How to Cite | Publication History | PlumX Article Matrix
Preparation of Tio2-PEG Thin Film on Hydrophility Performance and Photocurrent Response
Maulidiyah, Halimahtussaddiyah Ritonga, Catur Elok Faiqoh, Dwiprayogo Wibowo and Muhammad Nurdin*
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Halu Oleo, Kendari 93232 – Southeast Sulawesi, Indonesia
DOI : http://dx.doi.org/10.13005/bbra/1865
ABSTRACT: TiO2 photocatalytic process has been developed and used in various applications. One of the use of TiO2 is related to the hydrophilic properties, which is shown with a small contact angle between the surface of object and liquid (<10°). In this study, TiO2 was coated on a glass surface with the dip coating method. TiO2 catalyst was prepared by titanium tetraisopropoxide (TTIP) as precursor and polyethylen glycol (PEG) as template calcined at a temperature of 450°C. The addition of PEG was varied to obtain optimal conditions. Measurement of Diffuse Reflectance Spectroscopy UV-Vis (DRS UV-Vis) showed a decrease in the value of the band gap for TiO2, TiO2-PEG 200, TiO2-PEG 400 and TiO2-PEG 1000 were 3.153 eV; 3.056 eV; 3.012 eV and 2.975 eV, respectively. Results of contact angle measurements have been achieved hydrophilic properties. While, the photocurrent response of the three TiO2 working electrodes added the PEG 200, PEG 400, PEG 100 were 2.3×10-3 A, 2.2×10-3 A and 4.2×10-3 A, respectively. Therefore, PEG could improve the performance of TiO2 catalysts.
KEYWORDS: TiO2-PEG; Thin film; Hydrophilic; Photoelectrocatalytic
Download this article as:| Copy the following to cite this article: Maulidiyah, Ritonga H, Faiqoh C. E, Wibowo D, Nurdin M. Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Halu Oleo, Kendari 93232 – Southeast Sulawesi, Indonesia. Biosci Biotech Res Asia 2015;12(3) |
| Copy the following to cite this URL: Maulidiyah, Ritonga H, Faiqoh C. E, Wibowo D, Nurdin M. Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Halu Oleo, Kendari 93232 – Southeast Sulawesi, Indonesia. Biosci Biotech Res Asia 2015;12(3). Available from: https://www.biotech-asia.org/?p=2266 |
Introduction
TiO2 is a transition metal oxide which is chemically inert and stable. TiO2 has three kinds of crystal structures, namely anatase, rutile and brookite [1]. Anatase type of TiO2 is more photoactive than rutile because the surface area of anatase is greater than the rutile. So that the active site per unit of anatase is greater than rutile. Brookite structure is the most unstable and most difficult to prepare so it is rarely used in the process of photocatalyst [2].
The preparation of semiconductor thin film is one way to ease the semiconductor applications as a solar cell and photocatalyst in the degradation of harmful chemical compounds [3,4]. Photocatalyst technology is a combination of integrated photochemical and catalytic processes to be able to carry out a chemical transformation reactions. The transformation reaction takes place on the surface of a semiconductor catalyst materials induced by light [5].
Development in the preparation of TiO2 semiconductor electrochemically is a newer method by combining the photocatalyst with an electrochemical process, known as photoelectrocatalysis [6-8]. Nurdin and Maulidiyah reported in developing photoelectrochemical cell systems, the most important part is the preparation of the nano-sized TiO2 film [9]. Zhang et al. also reported the synthesis of mesoporous TiO2 using tetrabutilortotitanat as precursor as well as polyethylen glycol (PEG) as template [10]. The synthesis result of mesoporous TiO2 reported is having great surface area and photoelectrocatalytic activities. Many other studies have been conducted with various types of dopants, but dopant with PEG is more interesting than the other dopants, because PEG can improve the performance of the catalyst, and is able to increase the porosity, reduce the size of crystals and reduce the possibility of film cracking during the calcination process [11].
The photocatalytic process when the semiconductor absorbs light energy equal to or greater than the energy of its band gap, there will be a charge separation or photoexcitation in semiconductors. Electrons (e–) will be excited into the conduction band leaving positive hole (h+) in the valence band. The positive hole has a high affinity for oxygen in H2O molecules and form •OH radicals. Hydroxyl radical (•OH) is a highly reactive species that attacks organic molecules and can degrade into CO2 and H2O and halide ions if organic molecules containing halogen [7,12]. Therefore, the positive and negative holes on the activated titanium can then be used for various applications. One is for self-cleaning materials in a wide range using TiO2 [9].
One of important parameters from the contact angle is the influence of the surface structure, as reported by Xiong et al. the descend of water contact angle on the surface structure of TiO2 is caused by the formation of electron-hole, where the hole reacts with oxygen to form the surface of the empty oxygen and the electrons react with metal ions (Ti4+) to form ions (Ti3+) electron traps surface [13]. TiO2 affects the structure of a surface contact angle and photoelectrocatalytic activity. Several templates of TiO2 is friendly used for immobilization of TiO2, polyethylene glycol (PEG) that is often used to form pores for the synthesis of porous TiO2 films formed by hydrothermal method [14,15].
This research will develop a preparation method of the TiO2 catalyst by adding PEG dopant immobilized on Ti plate. Thus, it is expected to improve the performance of the catalyst, and is able to increase the porosity, reduce the size of the crystals and reduce the possibility of film cracking during the calcination process.
Research Methods
Synthesis of TiO2 Sol
TiO2 sol was prepared by mixing 35 mL of ethanol, 2.4 mL of diethanolamine (DEA), and 7.5 mL of titanium tetra isopropoxide (TTIP). The mixture was stirred at room temperature for 1.5 hours. The mixture was added ethanol: water (4.5 mL: 0.5 mL) and 0.5 mL of PEG 200 and stirred for 1.5 hours. With the same treatment and added 1 mL of PEG 400 and 2 mL of PEG 1000 and stirred for 1.5 hours.
Preparation of TiO2 Film
TiO2 sol was used to coat on a preparations of glass substrate with a dip-coating method, then calcined at 450°C for 2 hours.
Contact Angle Measurement
The contact angle of measured glass slide was placed at the back of the plate in a horizontal position. Then water was dripped using a syringe on the preparations surface. Further, the droplet images taken with a digital camera that light and magnification had been set up to obtain images of water droplets in focus. Furthermore, the image obtained was input into a computer to measure its contact angle with software protactor (MB-ruler). Then the contact angle was recorded as the surface contact angle before irradiated by UV. Finally, preparations were irradiated by 18 watt of UV for 1 to 4 hours in a UV reactor. After 1 hour irradiation, this process were repeated. Changes in the contact angle on the surface were compared with every hour irradiation.
Photocurrent Response
LSV was measured with Linear Sweep Voltammetry using 0.1 M NaNO3 solution, where 0.85 g of NaNO3 was dissolved in 100 ml flask. LSV testing was conducted on the potential -1 to 1 volt, with a scan rate of 1×10-4 V/s, in which each of TiO2 without PEG, TiO2-PEG 200, TiO2-PEG 400 and TiO2-PEG 1000 was tested by illuminating the UV-LED light. The LSV results were used to observe the photoelectrocatalytic activity during the testing process [16].
Results and Discussion
Determination of Band Gap Energy
Measurement with DRS UV-Vis aimed to determine the character of absorption in the wavelength range both for UV and Visible (200-600 nm). In Figure 1, it can be observed that for TiO2 with additional variations of PEG concentration, it has different reflectance spectrum profile. The reflectance spectrum profile clearly showed that the TiO2-PEG has an area of absorption in the visible light (λ> 400nm).
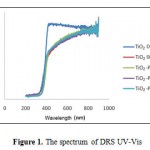 |
Figure 1: The spectrum of DRS UV-Vis |
Table 1: Values of Band Gap
| Sample | Band Gap (eV) |
| TiO2 Degussa | 3.4813 |
| TiO2 Standard | 3.1536 |
| TiO2-PEG 200 | 3.0565 |
| TiO2-PEG 400 | 3.0128 |
| TiO2-PEG 1000 | 2.9758 |
TiO2-PEG has band gap energy values smaller than TiO2 degussa and TiO2 standard. With the decrease in the band gap values, then the light energy needed by photohole and photoelectron will be smaller, simply by using a visible light source [7,16]. Moreover, it can also be seen that the higher concentration of PEG, it proved to have a tendency of decreasing the band gap values although the decrease was not significant, as can be seen on the Tabel 1 that the optimum band gap value was obtained at TiO2-PEG 1000 sol.
Measurement of Contact Angle (Hydrophilic Test of TiO2 -PEG Thin Film)
Hydrophilic test on TiO2 catalyst films was measured by contact angle meter. The contact angle measurements was performed by varying the exposure time of 20 Watt UV (0 to 4 hours) and varying the thin film of TiO2 which was added to the PEG. Figure 2 shows the contact angle without a thin film of TiO2 exhibits superhydrophobic wherein the contact angle is greater than 100°, so that the level of wetness is lower. Contact angle is relatively constant despite irradiated by UV light [17]. As for the contact angle measurements without PEG, PEG 200, PEG 400 and PEG 1000, were hydrophilic due to a decrease in the contact angle from 40° to 20°. These showed that the TiO2 film irradiated by UV light could transform into hydrophilic properties. Similarly, the length of time variation of UV radiation, the longer the exposure time, the intensity of UV rays on the surface of TiO2 was films getting bigger, so that the wetting was greater and contact angle of water would decrease.
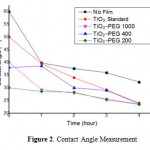 |
Figure 2: Contact Angle Measurement |
Measurement of Photocurrent Response
Figure 3 showed the photocurrent increased linearly at low potential and then saturated at higher potential. Photocurrent generated would be increased by the increasing concentration of organic compounds, it showed the increasing rate of photohole capturing, i.e the reacting hole with organic compounds on the surface of TiO2 film [18]. With the increasing rate of capturing photohole the charge recombination processes on the surface of TiO2 was reduced and the photocurrent value would increase. According to Maulidiyah et al. there are two factors affect the value of the photocurrent on the photoelectrocatalytic process, namely the transfer of electrons in the semiconductor layer and the capturing process of photohole on TiO2 or solution interface, these two factors are important in reducing the recombination of electron-hole pairs [16,19].
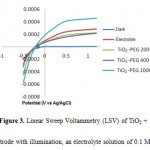 |
Figure 3: Linear Sweep Voltammetry (LSV) of TiO2 + PEG electrode with illumination, an electrolyte solution of 0.1 M NaNO3 |
The photocurrent was increased in Figure 3, the increase of the potential bias given at lower potential range when the organic compounds contained in the solution and showed the photocurrent saturation at a higher potential bias. Photocurrent saturation at a higher potential indicates the maximum rate of photohole capturing on the surface of TiO2. LSV response in Figure 3 shows that the three TiO2 working electrodes added to the PEG 200, PEG 400 and PEG 1000 were photocurrent measurements showed of PEG 200 generated of 2.3×10-3 A, PEG 400 the photocurrent generated was not too far from PEG 200 ranging of 2.2×10-3 A. While for PEG 1000 the surge in photocurrent generated was as high as 4.2×10-3 A showed that the activity of TiO2-PEG 1000 was better than the TiO2-PEG 200 and TiO2-PEG 400.
Conclusion
- The results of hydrophilic tests on the TiO2-PEG film had been reached the hydrophilic property.
- The photocurrent measurements value from three TiO2 working electrodes added the PEG 200, PEG 400 and PEG 1000 were 2.3×10-3 A, 2.2×10-3 A and 4.2×10-3 A, respectively.
Acknowledgement
We acknowledged the financial support of the Ditlitabmas-Dikti Ministry of Research, Technology and Higher Education, the Republic of Indonesia. We are also acknowledge to Surahman H. (Universitas Indonesia) for his valuable contribution in obtaining instrument measurements.
References
- Badawy, M.I., Souaya, E.M.R., Gad-Allah, T.A., Abdel-Wahed, M.S., Ulbricht, M. Fabrication of Ag/TiO2 photocatalyst for the treatment of simulated hospital wastewater under sunlight. Environmental Progress & Sustainable Energy, 2013; 33(3):886-8.
- Maulidiyah, Nurdin, M., Widianingsih, E., Azis T., Wibowo, D. Preparation of visible photocatalyst N-TiO2 and its activity on congo red degradation. ARPN Journal of Engineering and Applied Sciences, 2015; 10(15):6250-6.
- Dong, J., Han, J., Liu, Y., Nakajima, A., Matsushita, S., Wei, S. Defective black TiO2 synthesized via anodization for visible-light photocatalysis. Applied Materials & Interfaces, 2014; 6:1385-3.
- Wu, L., Li, F., Xu, Y., Zhang, J.W., Zhang, D., Li, G., Li, H. Plasmon-induced photoelectrocatalytic activity of Au nanoparticles enhanced TiO2 nanotube arrays electrodes for environmental remediation. Applied Catalysis B: Environmental, 2015; 164:217-7.
- Gupta, S.M., and Triphathi, M. A review of TiO2 Physical Chemistry (chinese Science), 2011; 56:1639-18.
- Nurdin,, Wibowo, W., Supriyono, Febrian, M.B., Surahman, H., Krisnandi, Y.K., dan Gunlazuardi, J. Pengembangan metode baru penentuan Chemical Oxygen Demand (COD) berbasis sel fotoelektrokimia: karakterisasi elektroda kerja lapis tipis TiO2/ITO. MAKARA Sains, 2009; 13:1-8.
- Nurdin, M. Preparation, characterization and photoelectrocatalytic activity of Cu@N-TiO2/Ti thin film electrode. International Journal of Pharma and Bio Sciences, 2014; 5(3):360-9.
- Maulidiyah, Ritonga, H., Salamba, R., Wibowo, D., Nurdin, M. Organic compound rhodamine B degradation by TiO2/Ti electrode in a new portable reactor. International Journal of ChemTech Research, 2015; 8(6):645-8.
- Nurdin, M. and Maulidiyah. Fabrication of TiO2/Ti nanotube electrode by anodizing method and its application on photoelectrocatalytic system. International Journal of Scientific & Technology Research, 2014; 3(2):122-4.
- Zhang, W., Li, R., and He, H. Synthesis of mesoporous TiO2-Al2O3 binary oxides photocatalyst by sol-gel method using PEG1000 as template. International Journal of Photoenergy, 2012; 2012: 1-7.
- Wang, C., Zhu, M., Liu, H., Cui, Y. and Chen, Y. A general method for mass and template-free production off hierarchical metal oxide spheres at room-temperature. RSC Advances, 2014; 4:24176-6.
- Feng, T., Feng, G.S., Yan, L., and Pan, J.H. One-dimensional nanostructured TiO2 for photocatalytic degradation of organic pollutants in wastewater. International Journal of Photoenergy, 2014; 2014:1-14.
- Xiong, L-B., Li, J-L., and Yu, Y. Ti3+ in the surface of titanium dioxide: generation, properties and photocatalytic application. Journal of Nanomaterials, 2012; 212:1-13.
- Mejia, J.C.M., Angeles, L., Almanza, R. Synthesis and characterization of TiO2 porous films for heterogeneous photocatalysis. Computational Water, Energy, and Environmental Engineering, 2014; 3:36-4.
- Qiao, X., Yang, H., Chen, C., Lou, X., Sum, J., Chen, L., Fan, X., Zhang, L., Shen, Q. Synthesis of monodispersed SnO2 microspheres via solvothermal method. Procedia Engineering, 2014; 94:58-5.
- Maulidiyah, Nurdin, M., Wibowo, D., Sani, A. Nanotube titanium dioxide/titanium electrode fabrication with nitrogen and Ag metal doped anodizing method: performance test of organic compound Rhodamine B degradation. International Pharmacy and Pharmaceutical Sciences, 2015; 7(6):141-6.
- Kelly, P.J., West, G.T., Ratova, M., Fisher, L., Ostovarpour, S., Verran, J. Structural formation and photocatalytic activity of Magnetron sputtered titania and doped-titaniua coatings. Molecules, 2014; 19:16327-21.
- Wang, S-H., Wang, K-H., and Jehng, J-M. TiO2 thin film with variad prous structure applied on the degradation of methylene blue. Journal of the Chinese Chemical Society, 2013; 60(12):1457-5.
- Maulidiyah, Nurdin, M., Erasmus, Wibowo, D., Natsir, M., Ritonga, H., Watoni, A.H. Probe design of chemical oxygen demand (COD) based on photoelectrocatalytic and study of photocurrent formation at SnO-F/TiO2 thin layer by using amperometry method. International Journal of ChemTech Research, 2015; 8(1):416-7

This work is licensed under a Creative Commons Attribution 4.0 International License.





