How to Cite | Publication History | PlumX Article Matrix
Alireza Zakeri1, and Mohammad Javad Rasaee1*
1Department of Medical Biotechnology, School of Medical Sciences,Tarbiat Modares University,Tehran,Iran Corresponding author email:rasaee_m@modares.ac.ir
DOI : http://dx.doi.org/10.13005/bbra/2022
ABSTRACT: Strptococcusequi subsp. Zooepidemicus (S. zooepdemicus) is among the hyaluronic acid (HA) producing bacterial strains. Due to its pervasive applications in medicine and cosmetics, HA is an economically valuable product. Therefore, charactering a novel high yield HA producing strain seems to be industrially appealing. In this regard we characterized a novel strain of S. zooepdemicus using. Thereafter, the amenable carbon and nitrogen sources were determined and optimized using one factor at a time approach. To have the highest yields of HA production during the fermentation, Taguchi orthogonal arrays were used to design 9 experiments. Our results revealed that glucose (30g/L) and yeast extract (70g/L) are the best carbon and nitrogen sources respectively. Moreover, according to our results the optimum culture conditions regarding the temperature, DO, rotate speed and pH were 37⁰C, 50%, 300 rpm and 7 respectively. The prioritized order of factors affecting HA production were as: temperature>DO > rotate speed> initial pH.The 103.84% increase in HA production under the optimum culture medium and conditions approves the robustness of the adopted approach. Since the wild type strain demonstrated high intrinsic capability of HA production in optimum conditions, this novel strain could be employed for large scale production of HA.
KEYWORDS: S. zooepdemicus; Hyaluronic acid; Taguchi orthogonal arrays; Fermentation
Download this article as:| Copy the following to cite this article: Zakeri A, Rasaee M. J. Identification of Wild Type Streptococcus Zooepidemicus and Optimization of Culture Medium and Fermentation Conditions for Production of Hyaluronic Acid. Biosci Biotech Res Asia 2016;13(1) |
| Copy the following to cite this URL: Zakeri A, Rasaee M. J. Identification of Wild Type Streptococcus Zooepidemicus and Optimization of Culture Medium and Fermentation Conditions for Production of Hyaluronic Acid. Biosci Biotech Res Asia 2016;13(1). Available from: https://www.biotech-asia.org/?p=6645 |
Introduction
Strptococcusequi subsp. Zooepidemicus (S. zooepdemicus) is a member of lance fields serogroup C which is regarded as the archetypal species of the closely related species S.equi subsp. Equi(1). The identification of S. zooepdemicus is customarily based on biochemical and molecular typing schemes including lance field grouping and Polymerase Chain Reaction(PCR).S. zooepdemicusisahyaluronic acid (HA) producing strain (2). Hyaluronic acid (HA) is a high molecular weight linear polysaccharide composed of repeating units of D-glucuronic acid and N-acetylglucosamine linked by β (1-3) and β (1-4) glycosidic bonds; due to its unique physiological and biological properties such as high water-holding capacity, visco-elasticity, moisture retention capacity and bio- compatibility, HA finds a wide range of applications in the fields of medicine and cosmetics, including osteoarthritis treatment, ophthalmic surgery, plastic surgery, drug delivery, skin moisturizers, and wound healing (3-5). Given its pervasive applications, it could be easily extrapolated that HA is an economically valuable product; therefore, acquiring a high quality and quantity HA production source seems to be an imperative priority for biologists. In this regard, microbial fermentation of HA producing bacterial strains has emerged as a new alternative for HA production. The first commercially fermented HA was produced from S. zooepdemicus, which remains to be the prevailing strain of industrial HA production (6); it has been reported that S. zooepdemicus could ideally produce 6~7 g/L HA under the suitable culture conditions (2).
Adopting an appropriate fermentation strategy for HA production is necessary for an economically thriving fermentation process. Fermentation process development involves the determination of culture conditions and the establishment of the cultivation mode. The influential factors in cultural conditions include medium composition, temperature, pH, aeration and agitation. Several reports have been published delineating the optimum culture conditions for HA production (7). Optimization of culture media and cultivation conditions along with the selection of appropriate colony are the most important factors exploited to increase the HA production quantity and quality. Among the various cultivation conditions batch culture appeared to be a common method for HA production, consequently there are many studies concerning the optimization of culture conditions in batch culture (6).
Since no general medium works best for a new strain being tested, the optimization of the culture medium for the wild type strain seems inevitable. However, the best medium chosen from shake flask data can build a foundation for the large scale stirred tank cultivation, thus it’s essential to improve the HA production through the optimization of shake flask medium and cultivation conditions.
In the present study,using biochemical and molecular methods a wild type S. zooepdemicusstrain was identified andits ability to produce hyaluronic acid was evaluated. To develop an optimum medium composition and cultivation condition for HA production, the approach of one-factor-at-a-time was employed to find out the best media constituents such as carbon and nitrogen sources. Thereafter, the optimum cultivation conditions including temperature, rotation speed, pH and dissolved oxygen (DO) were investigated through Tagochi orthogonal array design. To the best of our knowledge, it’s the first study reporting the identification, HA production verification, medium optimization and fermentation optimization of a wild type S. zooepidemicus wild type strain found in Middle East.
Materials and Methods
Bacterial isolation
Wild type S. zooepdemicus was isolated from horse nasal cavities in Iran and streaked into tryptic soy agar (TSA) with 5% (v/v) of ox blood. The culture was incubated at 37⁰C for 24h.
Biochemical characterization
The resultant colonies were characterized by characteristic colony morphology, standard gram staining and the ability to ferment sugar sources. Assessing the biochemical ability of the colonies to ferment different sugar sources, the isolated colonies were cultured on trypticase soy agar plates with different sugar sources including salicin, sucrose, sorbitol, lactose, raffinose, inuline and trehalose.
Molecular characterization
Since characterization of the 16S rRNA is regarded as golden method for molecular identification of bacteria, a PCR was designed to amplify and sequence the 16S rRNA of the wild type Streptococcus zooepidemicus. At first the genomic DNA of the wild type S. zooepidemicus was extracted using standard CTAB method and confirmed on agarose gel electrophoresis. The PCR was carried out using unique primers (F-16S rRNA 5′-AGAGTTTGATCCTGGCTCAG -3′ and R-16S rRNA: 5′- ACGGCTACCT TGTTACGACTT -3′) and the genomic DNA as template. The PCR program was consisted of 1 cycle of 5 min at 95 °C, followed by 30 cycles of 95 °C (30 s), 58 °C (30 s), and 72 °C (3 min) and one final extension cycle of 72 °C (10 min). PCR reactions were done by pfu DNA polymerase. Then, Gel electrophoresis (1% agarose) was performed to analyze the presence of the amplified sequence and its size. To identify the exact sequence of the 16S rRNA gene, it was sequenced byMacroGen Company. The sequencing result was submitted as a quarry in NCBI nucleotide BLAST tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine the similarities of the 16S rRNA sequences in other bacterial strains. The search was performed against the nucleotide collection of the BLAST tool.
Medium preparation and cultivation
The characterized wild type S. zooepdemicus was used to conduct fermentation studies. Stock cultures were stored at –80ºC in tryptic soy broth (TSB) medium with 25% glycerol. All growth and production experiments were carried out within a 5L-bioreactor (Model KL-5L, Iran Tajhiz Ted Co. Ltd, Iran) with a working volume of 1.2 L. The medium was sterilized at 121°C for 15 min within a suitable autoclave. Glucose and Na2HPO4.12H2O solutions were autoclaved separately and mixed aseptically with other components after cooling.
Two loops of isolated Streptococcus zooepidemicus cells from a fresh slant culture were transferred into 50 ml of seed culture media and grown on a rotary shaker at 200 rpm and 37 °C for 15h. Then cultures were scaled up using a glass 5L-bioreactor. Agitation was provided by a six-bladed disk turbine. The pH was automatically controlled adding 5 M NaOH solution, while the temperature could be tuned by an internal bioreactor heater. The dissolved oxygen tension (DOT) studies were carried out with air or air supplemented with oxygen.
Measuring HA molecular weight, HA concentration and biomass
Cell growth rate was assessed measuring the optical density of the culture broth samples at 600 nm using a UV-spectrophotometer (Shimadzu, UV120-02, Japan). The viscosity of culture broth was measured using a capillary viscometer (Model Ubbelohde, Germany). Molecular weight of the produced HA was also determined using capillary viscometer. Each sample from the bioreactor were first incubated with a 10% volume of 5% (w/v) SDS for 10 min to separate the cells and liberate the capsular HA (8, 9). The culture broth was centrifuged at 4,000 rpm for 30 min. Following the cell removal,2 ml of ethanol was added to 1 ml of the supernatant from the culture broth and the solution was then refrigerated at 4 °C for 24hr to precipitate hyaluronic acid. The precipitate was recovered by centrifugation at 4,000 rpm for 30 min and precipitated again using the same procedure. Ultimately, the pellet was dissolved in double distilled water and used for HA concentration analysis. To measure HA concentration carbazole reaction was employed. To this end, disodium tetraborate solution, which was dissolved in sulfuric acid and boiled for 15 minutes, was added to HA solution. After cooling to room temperature, carbazole solution was added and heated in water bath for 15 minutes; thereafter, the solution was cooled to room temperature and its OD was read at 530 nm with D-glucuronic acid as the standard (10). The data obtained from the monitoring of the growth rate was used to calculate the biomass. The measured optical density at 530 nm was converted to biomass using the following equation:
Biomass (g/L) = OD530× 0.26 ± 0.01 (11).
Optimization of culture medium
Optimization of the culture medium was carried out to find the best carbon and nitrogen sources to have the highest HA production rate for the wild type S. zooepidemicus. Using one-factor-at-a-time approach which is based on the single factor experiments, the optimum carbon and nitrogen sources were determined. The carbon source assuring the highest HA production rate was obtained by substituting the glucose with seven different carbon sources including viz. sucrose, fructose, xylose, maltose, lactose, starch and galactose. All carbon sources were used at 5% concentration (w/v). On the other hands, the nitrogen source assuring the highest HA production rate was obtained by substituting the yeast extract with different organic and inorganic nitrogen sources. The organic nitrogen sources included peptone, gelatin, soya peptone, meat peptone, mycological peptone, beef extract and corn steep liquor, while the inorganic nitrogen sources were potassium nitrate, di-ammonium hydrogen orthophosphate, sodium nitrate and ammonium nitrate. All nitrogen sources were used at 0.5 % concentration (w/v). The rest of additives were not evaluated because of their low amount and less impacton the HA production.
Adjusting the concentration of carbon and nitrogen sources
Based on previous studies the concentration of the carbon (glucose) and nitrogen (yeast extract) sources were selected from the least to the greatest amounts reported. Seven glucose concentrations ranging from 30 to 90 (g/l) (30, 40, 50, 60, 70, 80 and 90g/l) and six different yeast extract concentrations ranging from 10 to 60 (g/l) (10, 20, 30, 40, 50 and 60 g/l) were used to find the optimum amount of carbon and nitrogen sources for the highest HA production rate.
Kinetic analysis of batch culture
The kinetics of HA production by the wild type S. zooepidemicus was evaluated analyzing the HA concentration and the biomass. The samples were collected every hour before and after culture optimization.
Optimization of culture condition
Four main fermentation factors including temperature (33, 35 and 37 °C), rotation speed (100, 200 and 300 rpm), DO (20, 50 and 80 %) and original pH (7, 8 and 9) were evaluated regarding their effect on HA production yields. The Taguchi orthogonal array of the MATLAB software was employed to design the experiments for three levels of 4 selected factors. In each case, the yields of HA production was monitored and all of the experiments were carried out in triplicate.MINITAB 17 statistical software was used to create the main effect and S/N ratio plots by plotting the characteristic average for each factor level.
Results
S.zooepidemicus characterization
Culturing the isolated samples, typical β-hemolytic streptococci-like colonies were detected on blood agar.Among the different sugar sources the isolated colonies survived on TSA media with sorbitol and lactose as sugar source (12). Moreover, Genomic DNA of the wild type S. zooepidemicus was successfully isolated and observed on gelelectrophoresis.The PCR reaction using unique primers resulted in a 1300-bp DNAand observed on gelelectrophoresis (Figure1) .The sequencing results of the 16s rRNA gene indicated a high similarity to other known 16s rRNA sequences (figure 2). The BLAST search results revealed that the sequence of the wild type S. zooepidemicushas>87% of sequence match with the H70, CY, ATCC35246, 4047 and MGCS strains.
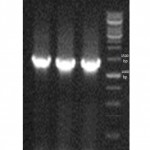 |
Figure 1: 16s rRNA PCR product. Typical PCR products of wild type S. zooepidemicuswith sizes of approximately 1300 bp amplified using the 16s rRNA- oligonucleotide primers. All lanes in the electrophoresis pattern indicates the different wild type S. zooepidemicus colonies. |
 |
Figure 2: The complete sequence of 16S rRNA of wild type S.zooepidemicus. The sequence indicates over 80% of identity with the 16S rRNA sequence of other S. zooepidemicus strains. |
Culture media preparation and optimization
The culture media was successfully prepared (as described under section 2.4) and the bacterial culture was done without any contaminations. Figures 3 and 4 indicates the HA production results, using various carbon and nitrogen sources to grow wild type S.zooepidemicus. Moreover, Figure 3a and 3b reveal the optimum concentrations of the selected carbon and nitrogen sources to have the highest HA production rate respectively. Apparently glucose (70 g/l) as carbon source and yeast extract (30 g/l) as nitrogen source have brought about the highest HA production yields (Figure 4).
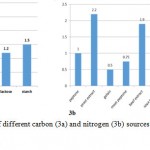 |
Figure 3: The effect of different carbon (3a) and nitrogen (3b) sources on HAproduction by wild type S.Zooepidemicus. |
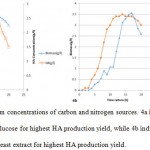 |
Figure 4: The optimum concentrations of carbon and nitrogen sources. 4a indicates the optimum concentration of the glucose for highest HA production yield, while 4b indicates the optimum concentration of the yeast extract for highest HA production yield. |
Kinetic analysis of the batch culture
Batch fermentation of wild type S. zooepidemicus in TSB medium was conducted before and after medium optimization with pHcontrolled at 7. As illustrated in Figure 5a in the case of fermentation before medium optimization, after 2 h of lag phase, cells started an exponential growth to 10 h and the biomass concentration reached the highest value at14 h, HA concentration reached the maximal value of 2.6 g /l at 15 h. Batch fermentation of wild type S. zooepidemicus in optimized medium was conducted and the results were shown in Figure 5b. HA and biomass concentration reached the maximal value of 4 g /l and 3.5 g/lrespectively at 14 h.
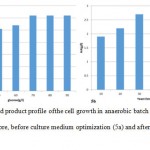 |
Figure 5: Growth and product profile of the cell growth in anaerobic batch culture for wild type S.Zooepidemicus before, before culture medium optimization (5a) and after culture medium optimization (5b). |
Table 1: Taguchi Orthogonal design and responsive values for HA production. The effect of temperature, rotation speed, pH value and DO are considered within all nine design experiments.
| Run | Temperature | Rotate speed | pH value | DO (%) | output of HA |
| (⁰C) | (RPM) | (g/l) | |||
| 1 | 33 | 100 | 7 | 20 | 1.7 |
| 2 | 33 | 200 | 8 | 50 | 2.1 |
| 3 | 33 | 300 | 9 | 80 | 2.4 |
| 4 | 35 | 200 | 7 | 80 | 2.8 |
| 5 | 35 | 300 | 8 | 20 | 2.4 |
| 6 | 35 | 100 | 9 | 50 | 3.1 |
| 7 | 37 | 300 | 7 | 50 | 5.3 |
| 8 | 37 | 100 | 8 | 80 | 4.1 |
| 9 | 37 | 200 | 9 | 20 | 3.4 |
Optimization of culture condition
Results of nine designed Taguchi orthogonal array experiments are presented in Tables 1, 2 and 3 along with figures 6. It’s demonstrated that the maximum HA production yield was 5.3 g/L. The optimum culture conditions regarding the temperature, DO, rotate speed and pH were37 (⁰C), 50 (%), 300 (rpm) and 7 respectively. Tables 2 and 3 demonstrated that the prioritized order of factors affecting HA production wereas: temperature>DO > rotate speed> initial pH.
 |
Figure 6: Main effects plot for S/N ratio (6a) indicating the effect of each factor in HA production yields, main effects plot for means (6b) indicating the best conditions to maximize the response, the steeper is the slope of the line, the greater is the magnitude of the main effect. |
Table 2: The HA production response for means.
| level | T | pH | RPM | DO |
| 1 | 2.067 | 3.267 | 2.967 | 2.500 |
| 2 | 2.767 | 2.867 | 0.400 | 3.500 |
| 3 | 4.267 | 2.967 | 3.367 | 3.100 |
| delta | 2.200 | 0.400 | 0.600 | 1.000 |
| rank | 1 | 4 | 3 | 2 |
Table 3: The HA production response for signal to noise ratios defined as the larger is better
| level | T | pH | RPM | DO |
| 1 | 6.219 | 9.346 | 8.897 | 7.614 |
| 2 | 8.792 | 8.768 | 8.672 | 10.252 |
| 3 | 12.457 | 9.354 | 9.898 | 9.601 |
| delta | 6.238 | 0.586 | 1.226 | 2.638 |
| rank | 1 | 4 | 3 | 2 |
Discussion
S. zooepidemicus is conventionally identified and differentiated by its cultural and biochemical properties. Therefore, our rudimentary knowledge of the isolated strain could be augmented by elucidating its cultural and biochemical properties. A positive lactose and sorbitol reaction (12) along with gram positive typical β-hemolytic streptococci-like colonies were the first clues confirmed that the isolated colonies belong to S. zooepidemicus. Moreover, the analysis of 16S rRNA sequence is regarded as a new method that can be used for bacterial identification in contemporary biology. A PCR-mediated identification by using species-specific segments of the 16S rRNA gene had already been used for identification of various streptococcal species (13-15). A large degree of identity (> 85%) among the 16s rRNA sequences of the wild type strain and the previously reported S. zooepidemicus strains could be construed as a proof to categorize the isolated wild type as a S. zooepidemicusstrain. The production conditions of heterotrophic cultures, like fermentation, could be easily controlled to achieve a high cell density. In turn, high cell density could greatly cuts down fermentation time, space and more importantly the cost of down-stream processing. During fermentation, the carbon source act as the major constituent to provide energy, build the cellular material and also increase the HA production yield. Nitrogen as the other element which its source should be taken into consideration is primarily important due to its structural roles within the main components of the cells. To delineate the influence of different types of carbon and nitrogen sources on the production of HA by wild type S. zooepidemicus, it was grown in glass 5L-bioreactor. There are various studies investigating the effects of carbon sources on HA production rates. Zhang J. et al. reported that the carbon sources like starch, lactose, dextrin and sucrose which are similar to glucose can be used for HA production (16). On a separate study Chong et al. reported the maltose (20 g/L) as the carbon source of S. zooepidemicus(ATCC 35246) batch fermentation (3). Armstrong &Johns used glucose (60 g/L) as carbon sources for S. zooepidemicus(ATCC 35246) (17) culture. To accumulate HA of high molecular weight in the culture mixture, Shibata et al. (18) reported the production of HA in a medium containing glucose and fructose as major carbon sources. Ranga swamy et al. (19) used sucrose and lactose as the best carbon source that the organism could utilize for HA production . However, our experiments showed that glucose and yeast extract resulted the highest production rate of HA. Although, the culture was able to grow in all carbon sources (sucrose, fructose, lactose and starch), individual experiments showed that glucose is the best carbon source tested.
The production of HA requires optimal amount of carbon and nitrogen sources for maximal production. In this study different types of nitrogen sources (organic and inorganic) were evaluated for their effect on growth and production of HA. Lee G. Y. et al. (20) and Chen Y. H. et al. (21) introduced yeast extract and beef extract as the nitrogen source of their respective fermentation process to produce HA. In line with the study of the Lee G. Y. et al. (given the glucose (70g/l) as the carbon source) the maximum HA amount of 4 g/l was achieved with yeast extract (30g/l) as the nitrogen source. The kinetic analysis of the wild type S. zooepidemicus batch culture revealed that the amount of HA production without optimized medium (2.6 g/l) is very low in comparison to optimized medium (4.1 g/l). The obtained results suggests that the optimization process could drastically enhanced the HA production yield and every novel strain could have its specific culture requirements.
Armstrong and Johns observed a 20% increase in HA titer when S. zooepidemicus were grown under aerobic conditions (22). Johns et al. also reported that the aerated culture gave higher HA concentration and yield than the equivalent anaerobic fermentation (23). Therefore, we chose the aerated batch culture condition for HA production. The system design, the parameter design and the tolerance design are the three steps of the Taguchi approach proposed for engineered optimization of a process (24). The Taguchi method aims to study a large number of variables with a small number of experiments exploiting orthogonal arrays. Thus, in order to determine the best fermentation conditions conducting the least number of experiments, Taguchi method was used for experiment design. The effects of agitation speed, aeration rate, shear stress, and dissolved oxygen on microbial HA production have been extensively studied (23,25,26). Moreover, Johns MR et al. evaluated the effect of pH, agitation and aeration on hyaluronic acid production by S. zooepidemicus(25). Regarding the previous reports we chose the temperature,pH, rotation speed and OD to be the factors of experiment design.
The effect of a variable taken in isolation is known as the main effect. In other words, a main effect is the effect of one of the independent variables on the dependent variable, ignoring the effects of all other independent variables. Using main effect it would be possible to essentially assess the overall effect of a given factor. It should be underpinned that, whenever different levels of a factor affect the response differently there is a main effect.A main effects plot graphs the response mean for each factor level connected by a line. When the plot line is horizontal or parallel to x-axis, then there is not a main effect. However, if different levels of the factor affect the response differently the plot line is not horizontal and there is main effect. The main effects plots of all evaluated factors were sloped, hence there were main effects for all of them. This means that each evaluated factor exert some effect in HA production yields and the best settings of each factor could be extracted from the available data. The experimental results could be transformed into a signal-to noise (S/N) ratio. The signal-to-noise ratio measures the variations of the desired response relative to target value under different noise conditions. If the signal-to-noise ratios are defined as large is better, the goal of the experiment is to maximize the response. Since we tried to find the qualified conditions for HA production the signal-to-noise ratios were defined as large is better to get the S/N ratio plots. Therefore, using these S/N ratio plots the best amounts of each variable factor was determined to augment the HA production yields. In this regard, the finally optimized culture components and conditions reached the highest level of 5.3 g/L HA production which seems to be promising yield for large scale and industrial production of HA. The production level of the HA could be increased even more applying metabolic engineering approaches. Targeting the metabolic pathway of the HA production within this novel strain would staggeringly enhance the production yields.
Conclusion
In conclusion it should be noted that characterizing a novel strain, finding the best components of its culture medium and ultimately introducing the best conditions for its fermentation seems to be an integrated strategy to prepare a microbial source for large scale production of a commercially significant product. The 103.84% increase in HA production under the optimum culture medium and conditions approves the robustness of the taken approach. Since the wild type strain demonstrated high intrinsic capability of HA production, the production levels could be enhanced applying metabolic engineering.
Acknowledgement
The authors wish to thank TarbiatModares University for supporting the conduct of this research. The authors declare that they have no conflict of interest.
Conflict of Interest
The authors declare no conflict of interests.
References
- Timoney JF. The pathogenic equine streptococci. Veterinary research. 2004;35(4):397-409.
- Liu L, Liu Y, Li J, Du G, Chen J. Microbial production of hyaluronic acid: current state, challenges, and perspectives. Microbial cell factories. 2011;10:99.
- Chong BF, Blank LM, McLaughlin R, Nielsen LK. Microbial hyaluronic acid production. Applied microbiology and biotechnology. 2005;66(4):341-51.
- Kogan G, Soltes L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnology letters. 2007;29(1):17-25.
- Kim SJ, Park SY, Kin CW. A novel approach to the production of hyaluronic acid by Streptococcus zooepidemicus. Journal of Microbiology and Biotechnology. 2006;16(12):1849-55.
- Krahulec J, Krahulcova J. Increase in hyaluronic acid production by Streptococcus equi subsp. zooepidemicus strain deficient in beta-glucuronidase in laboratory conditions. Applied microbiology and biotechnology. 2006;71(4):415-22.
- Jannatabadi AA, Mohammadi GR, Rad M, Maleki M. Molecular identification of Streptococcus equi subsp. equi and Streptococcus equi subsp. zooepidemicus in nasal swabs samples from horses suffering respiratory infections in Iran. Pakistan journal of biological sciences : PJBS. 2008;11(3):468-71.
- Chong BF, Nielsen LK. Amplifying the cellular reduction potential of Streptococcus zooepidemicus. Journal of biotechnology. 2003;100(1):33-41.
- Chong BF, Nielsen LK. Aerobic cultivation of Streptococcus zooepidemicus and the role of NADH oxidase. Biochemical engineering journal. 2003;16(2):153-62.
- Bitter T, Muir HM. A modified uronic acid carbazole reaction. Analytical biochemistry. 1962;4(4):330-4.
- Chen WY, Marcellin E, Steen JA, Nielsen LK. The role of hyaluronic acid precursor concentrations in molecular weight control in Streptococcus zooepidemicus. Molecular biotechnology. 2014;56(2):147-56.
- Alber J, El‐Sayed A, Lämmler C, Hassan A, Weiss R, Zschöck M. Multiplex polymerase chain reaction for identification and differentiation of Streptococcus equi subsp. zooepidemicus and Streptococcus equi subsp. equi. Journal of Veterinary Medicine, Series B. 2004;51(10):455-8.
- Hassan A, Khan I, Abdulmawjood A, Lämmler C. Evaluation of PCR Methods for Rapid Identification and Differentiation of Streptococcus uberis andStreptococcus parauberis. Journal of clinical microbiology. 2001;39(4):1618-21.
- Meiri-Bendek I, Lipkin E, Friedmann A, Leitner G, Saran A, Friedman S, et al. A PCR-based method for the detectionof Streptococcus agalactiae in milk. Journal of dairy science. 2002;85(7):1717-23.
- Khan IU, Hassan AA, Abdulmawjood A, Lammler C, Wolter W, Zschock M. Identification and epidemiological characterization of Streptococcus uberis isolated from bovine mastitis using conventional and molecular methods. Journal of veterinary science. 2003;4(3):213-24.
- Zhang J, Ding X, Yang L, Kong Z. A serum-free medium for colony growth and hyaluronic acid production by Streptococcus zooepidemicus NJUST01. Applied microbiology and biotechnology. 2006;72(1):168-72.
- Armstrong DC, Johns MR. Culture Conditions Affect the Molecular Weight Properties of Hyaluronic Acid Produced by Streptococcus zooepidemicus. Applied and environmental microbiology. 1997;63(7):2759-64.
- Shibata Susumu. KY, inventor Production of HA. Japan patent 06319579. 1994.
- Rangaswamy V, Jain D. An efficient process for production and purification of hyaluronic acid from Streptococcus equi subsp. zooepidemicus. Biotechnology letters. 2008;30(3):493-6.
- Lee G-Y, Ha S-J, Jung J-H, Seo D-H, Park J-Y, Kim S-R, et al. Effect of non-animal-derived nitrogen sources on the production of hyaluronic acid by Streptococcus sp. KL0188. Journal of the Korean Society for Applied Biological Chemistry. 2009;52(3):283-9.
- Chen Y-H, Li J, Liu L, Liu H-Z, Wang Q. Optimization of flask culture medium and conditions for hyaluronic acid production by a streptococcus equisimilis mutant nc2168. Brazilian Journal of Microbiology. 2012;43(4):1553-61.
- Armstrong DC, Johns MR. Culture Conditions Affect the Molecular Weight Properties of Hyaluronic Acid Produced by Streptococcus zooepidemicus. Applied and environmental microbiology. 1997;63(7):2759-64.
- Kim J-H, Yoo S-J, Oh D-K, Kweon Y-G, Park D-W, Lee C-H, et al. Selection of a Streptococcus equi mutant and optimization of culture conditions for the production of high molecular weight hyaluronic acid. Enzyme and Microbial Technology. 1996;19(6):440-5.
- Taguchi G. Introduction to Quality Engineering, Asian Productivity Organization (Distributed by American Supplier Institute Inc., Dearborn, MI). Dearborn, MI. 1986.
- Kotra SR, Venkateswarulu T, John B. Cost effective media optimization for the enhanced production of hyaluronic acid using a mutant strain Streptococcus zooepidemicus 3523–7: a statistical approach. Int J Adv Sci Technol. 2013;60:83-96.
- Liu L, Sun J, Xu W, Du G, Chen J. Modeling and optimization of microbial hyaluronic acid production by Streptococcus zooepidemicus using radial basis function neural network coupling quantum‐behaved particle swarm optimization algorithm. Biotechnology progress. 2009;25(6):1819-25.

This work is licensed under a Creative Commons Attribution 4.0 International License.





