How to Cite | Publication History | PlumX Article Matrix
Air Injection into the Stereotactic Biopsy Site in Cerebral Lesions; A Feasibility Study
Sohrab Shahzadi1, Bahram Hejrani2*, Alireza Zali1, Hamed Javadian3 and Khosro Parsa4
1Department of Neurosurgery, Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2Neurosurgeon, Fellow of Stereotactic and Functional Neurosurgery, Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3Resident of Neurosurgery, Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4Department of Neurosurgery , Iran University of Medical Sciences, Tehran, Iran.
Corresponding Author E-mail: b.hejrani@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2292
ABSTRACT: We assessed the utility and safety of injecting a small amount of air into the stereotactic biopsy site. Specifically, we wanted to know whether it helps in visualizing the target on a post-op brain CT scan. In this clinical series, we chose our subjects by consecutive sampling, among candidates for frame-based stereotactic biopsy of a supratentorial cerebral lesion. By applying certain inclusion and exclusion criteria, twenty cases were biopsied by two surgeons in a four-month period. After obtaining the tissue sample, we injected 0.5-1 ml of filtered room air through the biopsy cannula. The immediate post-op CT scan was evaluated by two researchers for the presence and location of the air bubble. The subjects consisted of 12 male and 8 female patients, 10-76 years old. The histologic diagnosis rate was 100%; 60% high – grade glioma, 20% low-grade glioma, 10% metastasis and one case of meningioma and brain abscess. The air bubble could be visualized in all cases within the lesion territory. In two cases (10%) bubbles were also found in other locations. There was no neurologic or radiologic complications. Our findings substantiates the limited available literature in regard with safety and diagnostic usefulness of air bubbles. Further research is needed to verify and quantify the diagnostic impact and any complications attributable to this measure.
KEYWORDS: Stereotactic Biopsy; Air Injection; Post-op CT Scan
Download this article as:| Copy the following to cite this article: Shahzadi S, Hejrani B, Zali A, Javadian H, Parsa K. Air Injection into the Stereotactic Biopsy Site in Cerebral Lesions; A Feasibility Study. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Shahzadi S, Hejrani B, Zali A, Javadian H, Parsa K. Air Injection into the Stereotactic Biopsy Site in Cerebral Lesions; A Feasibility Study. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15967 |
Introduction
Stereotactic surgery is a fixture in the modern neurosurgery armamentarium. Stereotactic biopsy of a cerebral lesion is a typical example of this approach (1,2). This intervention is commonly followed by a post-op brain CT Scan (BCT) (3,4) to rule out complications (5-7) such as hemorrhage and, if possible, ensure the accuracy of targeting. In this study we evaluated the safety and utility of injecting a small amount of air into the biopsy site through the biopsy cannula. The presumptive benefits were twofold. Firstly, it could help as a marker on the post-op BCT to “visualize” the biopsy site. Secondly, it might enhance hemostasis by creating a small local pack.
In this feasibility study, we present twenty cases in whom we injected a small amount of filtered room air following a standard stereotactic biopsy of their brain lesion.
Despite extensive search, we could not locate any similar study. Nevertheless, pneumocephalus is a well-described clinical entity, mostly as a complication of head injury or cranial surgery which per se mandates no treatment unless it causes significant mass effect i.e. tension pneumocephalus (8-10).
Zhi-jun SONG et al. described a similar technique in their study to assess the clinical efficacy of 1H-MRS and intraoperative MRI navigation in frameless biopsy of intracranial lesions (11). However the study is not aimed at evaluating the consequences of air injection.
They mention in their method:
“… After the biopsy, we routinely injected 0.5-1 ml of air to the target zone through the biopsy needle. It can either serve as a marker and confirm that the targeted tissue has already been acquired, or form an air plug for local hemostasis.” In the results section they continue:
“… None of the patients suffered from significant complications, such as intracranial bleeding or new neurologic deficit.” Therefore, it could be safely inferred that the air injection has also been uneventful.
In their classic textbook on stereotactic and functional neurosurgery, Lozano et al. allude to the usefulness of the presence of the air bubble in evaluating the biopsy site (12).
“… [Following the stereotactic biopsy] After removal of the stereotactic frame and base frame, a head CT without contrast is obtained. This allows verification of the biopsy target, typically by demonstrating a small amount of air at the previously identified target site”.
Material and Methods
The sampling method was consecutive sampling; all potential candidates, as determined by inclusion and exclusion criteria, were included. Surgeries were done by two senior neurosurgeons.
Included subjects were patients with a supratentorial lesion(s) in whom stereotactic biopsy had been contemplated. The latter phrase implied that the patient was eligible for the intervention (medically, legally and ethically) and other interventions such as upfront resection were not the obvious first choice (13).
From the above–mentioned candidates, we excluded two groups:
1-The cases in which the lesion lay within or was in contact with a vital structure such as brainstem, hypothalamus or a major vessel (because of the potentially devastating consequences of the mass effect).
2-The cases in which bleeding through the cannula was observed during the biopsy procedure (in order not to add to the volume of a probable hematoma).
After obtaining the biopsy specimen, in accordance with the standard technique (14), we left the cannula in place for a while to check for bleeding. In subjects selected for air injection, before removing the cannula, we injected 0.5-1 cc of filtered air room. The filtration process consisted of passing the air through a multilayered sterile gauze to remove any dust or particles.
After air injection, we kept the syringe connected for about ten seconds and then removed it and immediately inserted the stylet into the cannula. Then we gently removed the cannula and stylet together. The rest of the procedure continued as usual. Following the routine of our center, an immediate post-op BCT without contrast was obtained.
This BCT was evaluated to determine the presence and site of the air bubble. The result was independently checked by another researcher. In the case of disagreement, the topic was discussed until they reached a consensus.
Results
Our subjects consisted of twenty cases of supratentorial lesions who underwent stereotactic biopsy in the four-month period between November 2015 and April 2016. The findings are detailed in table 1.
Table 1 : Subjects’ demographic and clinical information and air injection results
| Number, Name | Gender, Age | Target Location | Presentation | Histologic Diagnosis | Bubble Location | |
| Within the lesion | Other locations | |||||
| 1-H.J. | M 59 | Lft F | behavioral changes | GBM | + | |
| 2-P.A. | F 50 | Rt P | Lft hemiparesis | GBM | + | |
| 3-K.M. | M 26 | Rt P | seizure | LGG | + | |
| 4-Z.N. | F 60 | Lft O | headache | GBM | + | |
| 5-K.A. | F 72 | Lft P | dizziness | GBM | + | |
| 6-M.A. | M 52 | Lft P | Rt hemiparesis | GBM | ||
| 7-F.J. | F 59 | Rt F | headache | Metastasis | + | |
| 8-O.H. | M 60 | Rt P | seizure + hemiparesis | Abscess | + | |
| 9-F.M. | M 76 | Lft P | Rt hemiparesis + aphasia | Metastasis | + | |
| 10-M.A. | M 67 | Rt Thalamus | Lft hemiparesis | HGG | + | |
| 11-H.P. | M 21 | Rt Thalamus | Lft hemiparesis | GBM | + | |
| 12-K.Q. | F 25 | Lft T | headache | LGG | + | + |
| 13-A.N. | M 42 | Lft F | impaired consciousness | GBM | + | |
| 14-M.R. | F 10 | Lft P | seizure | Pilocytic Astrocytoma | + | |
| 15-A.S. | M 25 | Lft Thalamus | behavioral changes | GBM | + | |
| 16-M.R. | M 27 | Rt T | seizure | LGG | + | |
| 17-D.H. | M 45 | Lft P | Rt hemiparesis | HGG | + | |
| 18-S.N. | M 23 | Lft P | Rt hemiparesis + seizure | GBM | + | |
| 19-A.Q. | F 58 | Lft TP | headache | HGG | + | + |
| 20-M.B. | F 40 | Rt Atrium | headache | Meningioma | + | |
Demographically, the subjects’ age range was 10-76 years. 12 (60%) were male and 8 (40%) were females. The diagnostic yield was 100% and final histologic diagnoses were as follows:
High–grade (III or IV) glioma; 12 cases (60%), 9 harboring GBM.
Low–grade (I or II) glioma; 4 cases (20%) including one case of pilocytic astrocytoma.
Metastasis; 2 cases (10%)
Others; abscess and meningioma, one case (5%) each.
In all cases, the air bubble was easily recognizable on post-op BCT (Figure 1).
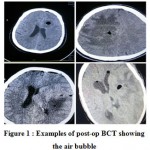 |
Figure 1: Examples of post-op BCT showing the air bubble.
|
We arbitrarily described the bubble location as either:
“Within the lesion”; air bubble seen within the area of signal change on MRI.
“Other locations”; such as intraventricular, in basal cisterns or cortical subarachnoid space, in the brain parenchyma beyond the lesion border, etc.
Obviously, a BCT could accommodate bubbles of both types (which happened in two cases, the bottom photos in Figure 1).
The only exception to this designation was that we ignored a small (less than 2 cc) pneumocephalus at the burr hole site or frontal subdural space which was clearly attributable to burr hole and irrigation (e.g. top left photo in Figure 1).
The two researchers in charge of BCT evaluation were in reasonable agreement in all cases about the existence and location of the air.
Describing the bubbles thusly, all cases had air bubbles within the lesion which was deemed to be the biopsy site
Two cases (10%) had bubbles in other locations too; case no.12 (air in neighboring brain parenchyma, Figure 1 bottom left) and case no.19 (intraventricular air, Figure1 bottom right).
We monitored the patients clinically by serial neurologic examinations for about 48h which is the usual length of hospital stay after stereotactic biopsy in our center. None of the patients developed new neurologic deficits following the intervention.
Discussion
In our series, the idea of injecting air into the biopsy site proved to be safe and diagnostically helpful. This finding corroborates the limited available literature on this topic. To the best of our knowledge, this feasibility study is the first of its kind. However, this issue has been obliquely addressed by at least two other groups (11,12) , while describing their method of stereotactic biopsy of cerebral lesions. While admitting its usefulness (in determining the site of biopsy taking and perhaps hemostasis), neither mentioned any complication attributable to the air in the brain. We used similar amount of air as described by Zhi-Jun SONG et al. (0.5-1 cc) (11).
In theory, this intervention could pose added risks through several mechanisms; increasing ICP, introducing infective microorganisms, causing neural damage by distorting or dissecting through neural fibers, impairing the perfusion of the surrounding parenchyma, etc. However, none of these phenomena have been observed in practice.
We acknowledge that the subjective nature of the data presented is a drawback to this research; deciding the presence, locality and volume of the air was mostly based on personal judgement. Nonetheless, this methodology sounds appropriate for a pilot study. To reduce the error, two researchers evaluate the BCTs independently.
In each and every step of the research, we adhered to the ethical code in medicine (15).
Written and informed consent was taken.
Based on the available literature, there was no grounds to believe that the procedure incurs any harm to the patient. However, as described in the method, we took an additional precaution by excluding certain patients that were regarded as “high risk”
We kept the identity of the patients confidential.
Looking ahead, we propose to quantitatively evaluate the diagnostic impact of this measure and its potential role in hemostasis or any other effect (positive or negative) on patient’s condition. But above all, one must stick to the maxim “Primum non nocere” thus, assessing the safety of this intervention must be high on the agenda. Clearly, deriving these information warrants more advanced methodologies involving experimental design.
Acknowledgement
The authors wish to express heartfelt gratitude to their colleagues in Shohada Tajrish Hospital operating room, Ms. Mohammad Hossaini, Ms. Goodarzi, Ms. Jahanabadi Mr. Shabani and Ms. Khankeshloo for their invaluable help in conducting the operations and research.
References
- Richard Winn H. Youmans Neurological Surgery. 6th Edition 2-Volume Set. Oxford University Press ; 2012. pp.1254-59.
- Schmidek & Sweet. Operative Neurosurgical Techniques. 5th Edition 1-Volume Set. Elsevier Press ; 2006. pp.625-29.
- Schmidek & Sweet. Operative Neurosurgical Techniques. 5th Edition 1-Volume Set. Elsevier Press ; 2006. pp.630-34.
- Lozano AM. Textbook of Stereotactic and Functional Neurosurgery. 2nd Edition 1-Volume Set. Springer‐Verlag Press ; 2009. pp 649-53.
CrossRef - Schmidek & Sweet. Operative Neurosurgical Techniques. 5th Edition 1-Volume Set. Elsevier Press ; 2006. pp. 630-34.
- Richard Winn H. Youmans Neurological Surgery. 6th Edition 2-Volume Set. Oxford University Press ; 2012. pp.1257-63.
- Lozano AM. Textbook of Stereotactic and Functional Neurosurgery. 2nd Edition 1-Volume Set. Springer‐Verlag Press ; 2009. pp 653.58.
CrossRef - Mark S. Greenberg. Handbook of Neurosurgery. 8th Edition 1-Volume Set. Oxford University Press ; 2016. Pp.88-92.
- Mark S. Greenberg. Handbook of Neurosurgery. 8th Edition 1-Volume Set. Oxford University Press ; 2016. pp. 889-92.
- Richard Winn H. Youmans Neurological Surgery. 6th Edition 4-Volume Set. Elsevier Press; 2012. pp. 3509-603.
- Zhi-jun SONG, Xiao-lei CHEN, Bai-nan XU, Guo-chen SUN, Jia shu ZHANG, Fang-ye LI et al. Proton Magnetic Resonance Spectroscopy and Intraoperative MRI Navigation for Frameless Biopsy. Journal of Neurological Sciences. 2012; 29(3): 467-475.
- Lozano AM. Textbook of Stereotactic and Functional Neurosurgery. 2nd Edition 1-Volume Set. Springer‐Verlag Press ; 2009. pp. 649-52.
CrossRef - Schmidek & Sweet. Operative Neurosurgical Techniques. 5th Edition 1-Volume Set. Elsevier Press ; 2006. pp. 627-30.
- Schmidek & Sweet. Operative Neurosurgical Techniques. 5th Edition 1-Volume Set. Elsevier Press ; 2006. pp. 628-29.
- Frank A. Riddick, Jr. The Code of Medical Ethics of the American Medical Association. Ochsner J. 2003; 5(2): 6-10.

This work is licensed under a Creative Commons Attribution 4.0 International License.





