How to Cite | Publication History | PlumX Article Matrix
Cytotoxicity Comparison of Different Common Bracket-Wires Combinations used in Orthodontic Treatment
Masood Feizbakhsh1, Razie Mirsafaei1*, Soosan Sadeghian1 and Mehrafarin Fesharaki2
1Department of Orthodontics, School of Dentistry, Islamic Azad University of Isfahan (Khorasgan) Branch, Isfahan, Iran.
2Department of Cell Sciences Research Center, Medical Sciences University of Isfahan, Isfahan, Iran.
Corresponding Author E-mail: Razimir62@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2337
ABSTRACT: Since metallic ions are vital for adverse biologic effects such as allergy, cytotoxicity, mutagenicity and carcinogenicity, the corrosion of alloys is the key importance of its biocompatibility. The purpose of this study was comparing the cytotoxicity generated by different combination of common orthodontic bracket and wire after 7, 15 and 30 days. The materials include 5cm of 16 2 inch stainless steel, nickel titanium and beta titanium wires were coupled to central incisor to second premolar stainless steel brackets. To examine cytotoxicity, human gingival fibroblasts directly exposed to different couples. Cell culture was added to the plate of 6 wells containing the specimens and incubated in 5% carbon dioxide at 37°C and cell viability for each pair corrosion products compared by MTT test. One-way ANOVA and RM ANOVA analysis were used and p value <0.05 was considered significant. The analysis showed different range of cytotoxicity between various combinations and days, respectively (P<0.05). Post-hoc pairwise comparison illustrated cytotoxicity decreased with time (P<0.001) and in comparison of different combinations, Beta-Titanium wire with Stainless steel bracket generates the highest cytotoxicity and had significant differences with stainless steel brackets-stainless steel wires (P=0.007) and nickel titanium wire and brackets was in between with insignificant differences (P=0.369 with Beta-Titanium combination and P=0.069 with stainless steel combination). Coupling titanium alloys with stainless steel alloy remarkably increased the corrosion of stainless steel alloy induced localized cytotoxic effects.
KEYWORDS: Archwires; Brackets; Galvanic corrosion; Orthodontic alloy
Download this article as:| Copy the following to cite this article: Feizbakhsh M, Mirsafaei R, Sadeghian S, Fesharaki M. Cytotoxicity Comparison of Different Common Bracket-Wires Combinations used in Orthodontic Treatment. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Feizbakhsh M, Mirsafaei R, Sadeghian S, Fesharaki M. Cytotoxicity Comparison of Different Common Bracket-Wires Combinations used in Orthodontic Treatment. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=16207 |
Introduction
The increasing role of metal alloys in dentistry and the effects of ion release on health have attracted the involvement of many research workers.(1,2) Despite numerous studies on alloys biocompatibility over the last 30 years , the questions raised don’t have definitive answers, confirming the demand for more research in this field.( 3)Orthodontic treatments are always benefiting from metallic alloys throughout the history of this science since founded by Kingsley in 1880.(4) These treatment periods may take up to several years and the appliances used would remain in the oral cavity within this period.(5) This moist environment results in this alloys corrosion.(6)The heterogenic nature of these alloys and utilization of different combinations of them in the oral cavity demands exceptional attention to their biocompatibility.(7) Combinaton of metal alloys in saliva turns the oral cavity into a complete corrosion cell that saliva acts as an electrolyte.(7) In this environment ,two alloys with different corrosion potentials, electrically being coupled to each other when placed in contact .(8,9) This causes galvanic corrosion in which one metal usually acts as an anode and the other a cathode, that is leading to preferential release of metal ions from the anode. (8, 9) In addition, temperature and PH fluctuations, enzymatic and bacterial activities, and various chemical introduced into the oral cavity are corrosion accelerators.( 7)Since metallic ions are vital for adverse biologic effects such as allergy, cytotoxicity, mutagenicity and carcinogenicity, (7) the corrosion of alloys is the key importance of its biocompatibility. (10)Various studies have evaluated the biocompatibility and adverse biologic effects of orthodontic appliances, and each has evaluated different parameters by using different approaches. Parameters such as ion concentration in saliva,(11) blood serum(12) or buccal mucosa cells(13) with spectrometry,(14,15,16) the galvanic current produced by alloys,(9) enzymatic mitochondrial activity(17) and genomic damage(18,19,20) have been assessed (Table 1).
 |
Table 1: previous and present study comparison
|
As a leading research group, Sarkar et al. (21) evaluated the corrosion of Stainless steel, Elgiloy, NiTi and Beta-Titanium wires using SEM microscope and X-ray. The result presented that Stainless steel, Elgiloy and Beta-Titanium are passive but NiTi is susceptible to pitting corrosion.
Cytotoxicity evaluation on fibroblast cells was done by Rose et al. (6) He evaluated the toxicity of 23 types of wires and reported that stainless steel wires should be used with caution.
Yonekura et al. (22) measured the concentration of the released ions from common orthodontic wires and the result indicated Beta-Titanium wire had the highest biocompatibility whilst Stainless steel, NiTi and Elgiloy wires should be used with caution.
Lijima et al. (8) measured the galvanic current between different combinations of wires and brackets, and showed Stainless-steel brackets used with NiTi wires had the highest galvanic current.
Hafez et al.(7) evaluated the concentration of nickel and chromium ions, and the genotoxicity and cytotoxicity of oral mucosa epithelial cells in orthodontic patient and showed that Stainless steel bracket in combination with Stainless steel wire had more cytotoxicity and Stainless steel bracket in combination with NiTi wire had more genotoxic effects.
The results, however, demonstrate contradictory findings and insufficient output data which results in confusing orthodontists when selecting biologically safe appliance for their patients.(3) Moreover, Based on state laws, it is becoming increasingly important to promote the patient awareness of adverse reactions side effects.( 23)
Based on the previous studies reviewed in this paper, direct placement of appliance in adjacent with cells is a different method to evaluate wire and bracket combination cytotoxicity. The main aim of this study is comparing the cytotoxicity of different combination of common orthodontic bracket and wires (Table 2), and the results of this method with others.
Material and Methods
Test materials
This study used three different bracket-wire combinations, which was experimented thrice per pair. The brackets (Gemini MBT Rx 0.022inch slot) consist of central incisor to second premolar stainless steel brackets and three types of orthodontic wire were including Stainless steel (permachrom resilient) and Nickel Titanium (Nitinol Super-Elastic) and Beta Titanium (Beta III Titanium) wire all with 0.016×0.022 inch cross sectional diameter and 5cm long. All material used were made by 3M/Unitek USA Minnesota.
Cell culture
The gingival biopsy was taken from 10-year-old female patient undergoing frenectomy. The tissue was washed three times in phosphate-buffered saline (PBS), then minced (10 to 20 mm) and digested in 3mg/ml collagenase I (Sigma Aldrich st, Louis, Missouri, Germany). After 60 minutes in a shaking water bath, the dispersed material was filtered through a nylon mesh with a pore size of 2 µm. After centrifugation 2000 rpm for 10 minutes, the pellet was resuspended in PBS and centrifuged 2000 rpm for another 10 minutes. The cells were plated in a 75cm2 flask, containing modified Eagle medium F-12 (DMEM/F-12) plus 10% fetal calf serum (FCS) and 1% penicillin/streptomycin, and incubated at 37 oC in a humidified 5% CO2 atmosphere. Until the cells were approximately 80% confluent, the culture media was refreshed every 3 days. The cells were routinely sub-cultured by trypsinization (0.05% [w/v] trypsin and0.02% [w/v] EDTA in PBS).
The approach
Sterilization was done by autoclave (Ecotec Irantolid) for 60 minutes. Fibroblast cells reached of more than 80% confluence were trypsinized and seeded 4000cells/well in 6 well plate for 48 h and then added three different wires coupled with stainless steel brackets (SS wire group, Niti wire group and B-Ti wire group). After7, 15 and 30 days, the cells viability were assessed and repeated 3 times. Microscopic images (10x) of fibroblasts on day 30 was prepared (figure 1).
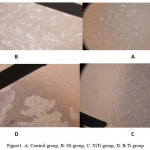 |
Figure 1: A) Control group, B: SS group, C: NiTi group, D: B-Ti group
|
Assay of viability and Cytotoxicity
The cells proliferation on different substrates was determined by using the colorimetric MTT (3-[4, 5-dimethythiazol-2-yl]-2, 5-diphenyl tetrazolium bromide) assay.
After7, 15 and 30 days of cells seeding (1×102 cells/well) in 6-well plate, cells were washed with PBS and then media were replace with a basal medium containing 0.005% MTT solution (400μl DMEM+40μl MTT). After 4 hour incubation, the medium was discarded. Then, the precipitated formazan was resolved using DMSO. The plates were incubated for 30 minute and aliquots of 100μl were transferred into a 6-well plate. The absorbance of each well was detected by Microplate reader (Binder made in Germany) at the wavelength of 540 nm.
Statistical Analysis
Cell viability was calculated according to the following formula:
Cell viability (%) = (optical density of test group/optical density of cellular control group) x 100
Three different analytical methods being used in this study to evaluate the statistics; RM ANOVA, ONE-WAY ANOVA and POST HOC LSD.
Results
Table 2 demonstrates the variation of means and standard deviations for different couples over time based on MTT test. As the table shows different bracket and wire couples have various cytotoxicity and this cytotoxicity decline with the time.
Table 2: MTT test mean value for different couples and days
|
|
7th day | 15th day | 30th day | Average |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Control group | 0.982 0.024 | 3.780 0.215 | 4.690 0.231 | 3.150 1.632 |
| SS wire group | 0.551 0.027 | 3.001 0.168 | 3.875 0.168 | 2.476 1.453 |
| NiTi wire group | 0.514 0.006 | 2.843 0.096 | 3.750 0.376 | 2.369 1.417 |
| B-Ti wire group | 0.425 0.027 | 2.973 0.165 | 3.351 0.236 | 2.316 1.406 |
The initial analysis carried out by RM ANOVA and ONE-WAY ANOVA is also supporting the MTT test results. A ONE-WAY ANOVA analysis showed significant differences in cytotoxicity between different combinations and RM ANOVA analysis showed significant differences between different days (P<0.05) .
Following above, POST HOC LSD analysis on the effect of time illustrated significant differences in cytotoxicity over days 7th to 15th and 15th to 30th in which cytotoxicity decreased with time (P<0.001).
In addition, POST HOC LSD presented the variation of cytotoxicity in different combinations which results has shown B-Ti wire group generates the highest cytotoxicity followed by NiTi wire group in the second place. Coupling the SS bracket and wire resulted in the least cytotoxicity (table 3).
Table 3: The result of materials pairwise comparison
| p- value | Groups |
| 0.069 | SS wire group,NiTi wire group |
| 0.007* | SS wire group,B-Ti wire group |
| 0.369 | NiTi wire group,B-Ti wiregroup |
Discussion
Systematic and local toxicity can be caused by ions released during the corrosion process in the mouth. There are many supportive documents emphasizing the occurrence of systemic toxicity whereas local toxicity deemed as higher risk and less focus of attention. The reason behind this risk is due to exposure of local tissues to the high concentrations of ions in the oral cavity. (10) Since chronic levels of metal ions can influence cellular function and even gene stability, general consensus that there is no concern around the corrosion by-products released in orthodontic patients is not actually confirmed .(7)
Different studies have examined the toxic effects of orthodontic appliances using various techniques while there is no consensus regarding these effects. Some studies have measured the ion concentration released by orthodontic appliances in body fluids and compared it with the systemic toxic threshold. Due to the permeability and excretory ratio of metallic ions investigation of ions in blood serum level is significantly complex.(1) Measurement of ion concentration in saliva, presented drawbacks such as momentary sampling, various secretion rates in different individuals, constantly washed and swallowed saliva, inability to measure the cumulative effects of ions absorbed by the surrounding tissue, and mix affiliation with salivary flow rate which influenced by factors such as time of day, health and diet.(1,7)
The cytotoxic and genotoxic effects also were investigated through either in-vivo or in-vitro studies by others. The In-vivo studies usually influenced by biologic variations introduced by each patient, which are not under experimental control, affect the standardization of the study and hence the difficulty in the interpretation of the results.(7) On the other hand, In-vitro studies are not flawless. They are pertinent to their lack of clinical relevance and simulation of the oral cavity. However, these studies are advantageous because they are simple to perform, providing a significant amount of information, and can be conducted under control conditions. (20) Therefore, this study was conducted by In-vitro approach and evaluated cytotoxicity and its relation to time and type of bracket-wire combinations.
The effect of time
Bishara et al. (12) study showed increase of blood ion level in treated patients after 2 and 4 months was not observed. Similarly in Kocadereli et al. (24) study the concentration of salivary ions in the first week, first and second months of treatment period was measured and demonstrated no increase. Agoaglu et al. (25) study has reported the highest amount of salivary ions in the first month while Hwang et al. showed the increase within a three months period. Petumeneu et al.(4) conducted that it increased immediately after appliance placement and decreased after 10 weeks.
The measurement of Nickel and Chromium ion concentration undertaken by Barret et al.(26) reported the increase in Nickel ion concentration within the first week and decline trend afterwards whereas increase within two weeks and decreasing thereafter for Chromium. Sahoo et al. (27) and Singh et al.(16) reported increase within the first week while decrease until day 30 and week three respectively.
The observation made within three different check points in the current study demonstrated the meaningful differences between days 7, 15 and 30 which indicates the increase in the viability of the cells in duration of examination period.
Apart from Bishara(12) and Kocadereli et al.(24) studies, others have come up with similar patterns in the way ion concentration increases for a short period and later decreases in saliva and blood. Barret et al. (26) described that the ion release on the surface of Stainless steel metal in the early stage is happening quickly and as a result, the release process declines. Moreover, the accumulation of corrosion product on the surface may also decrease the ion release level too.
Despite the similarity in the ion levels changes, ion release time differences are noticeable in the various studies. The comparison between this study and those conducted by Agoaglu, (25) Hwang (14) and Petumeneu et al. (4) is highlighting the timing variation of ion release timing. This means although the ion concentration progressively increased in day 30 and up to the third month in Agoaglu(25) and Petumeneu(4) studies respectively, there is no linear relationship between this concentration and the cytotoxicity because the cell’s adoption ability to the initial damage caused by metal ions prevents the progressive cytotoxicity. The result of study, however, demonstrated similar timing behavior in compare to Barret(26), Sahoo(27) and Singh(16) experiments.
The effect of combination type
Few studies have investigated toxic effect of orthodontic appliances when are in combination with each other. The result has shown the SS bracket in combination with B-Ti wire generates the highest cytotoxicity and coupling the SS bracket and wire resulted in the least cytotoxicity. NiTi wire and brackets was between them with insignificant differences.
At first glance, these results may contradict the general belief around the highest biocompatibility of Titanium alloys (22, 28) that is attributed to the high resistance of these alloys towards corrosion. However, this belief is true when such alloys are alone in a biologic environment, while contemporary orthodontics is based on the use of different combinations of alloys. So in such circumstances, the galvanic current influences the results of biocompatibility that is not necessarily consistent with the general belief.
The result of this study are consistent with Bakhtari et al.(9) study which has concluded the combination of B-Ti wire with any bracket produces the highest amount of galvanic current.
Yonekura et al. (22), who evaluated corossion characteristic of six orthodontic wires made of different alloys, stated that B-Ti wire has the highest general and localized corrosion resistance among the wires investigated. He suggested B-Ti is the most biocompatible wire. This is supporting the results of this study which emphasizes on the maximum cytotoxicity driven from B-Ti wire group because B-Ti wire has the highest corrosion resistance, acts as a cathode while the SS bracket plays the opposite role as the anode and experienced the most corrosion rate.
Lijima et al. (8) reported different results. He stated coupling of SS bracket and NiTi wire produced a higher galvanic current compare to the combination of SS bracket and B-Ti wire.
Among Titanium alloys, B-Ti has the most corrosion resistance so in combination with SS bracket generates the most cytotoxicity whereas NiTi alloy with SS bracket has less cytotoxicity and finally combination of SS bracket and wire has the lowest cytotoxicity.
The method of examination in this study that directly placed appliances beside the fibroblasts in cell culture, is more similar to the oral cavity in compare to the rest of In-vitro studies, could demonstrate the more realistic results.
Despite the fact that titanium alloys are known for their biocompatibility (28), the results illustrated that its combination with other alloys does not guarantee any biological changes. Due to the galvanic corrosion caused by orthodontic treatments, an increased corrosion-resistant of alloys would not count as an advantage. Therefore, this study suggests that the condition of biocompatibility evaluation must be examined in combination, similar to the orthodontic treatment not only bracket or wire individually.
Conclusions
We concluded that
Cytotoxicity decreased over time.
B-Ti wire in combination with SS bracket showed the most cytotoxic behavior.
Coupling the SS wire and bracket resulted in the least cytotoxicity.
References
- Eliades T, Partsinis H, Kletsas D, Eliades G, Makou M. Characterization and cytotoxicity of ions released from stainless steel and NiTi orthodontic alloys. AM j Orthod Dentofacial Orthop 2004; 125:24-9.
CrossRef - Costa MT, Lenza MA, Gosch CS, Costa I, Ribeiro –Dias F. In vitro evaluation of corrosion and cytoyoxicity of orthodontic brackets. J Dent Res 2007; 86:441-5.
CrossRef - De Souza RM, De Menezes Nickel, chromium and iron levels in the saliva of patients with simulated fixed orthodontic appliances.Angle Orthod 2008:78:345-50
CrossRef - Petoumenou E, Arndt M, Keilig L, Reimann S, Hoederath H, Eliades T, et al. Nickel concentration in the saliva of patients with NiTi orthodontic appliances. Am J orthod Dentofacial Orthop 2009; 135:59-65.
CrossRef - Tomakidi P, Koke U, Kern R, Erdinger L, Krüger H, Kohl A, et al.Assessment of acute cyto- and genotoxicity of corrosion eluates obtained from orthodontic materials using monolayer cultures of immortalized human gingival keratinocytes. J Orofac Orthop 2000;61:2-19
CrossRef - Rose EC, Jonas IE, Kappert HF. in vitro investigation into the biologic assessment of orthodontic wires. J Orofac Orthoped 1998; 59:253-64.
CrossRef - Hafez HS, Selim EM, Kamel Eid FH, Tawfik WA, Al-Ashkar EA, Mostafa YA. Cytotoxicity, genotoxicity, and metal release in patients with fix orthodontic appliance:A longitudinal in-vivo study. Am J Orthod Dentofacial Orthop 2011; 140:298-308
CrossRef - Lijima M, Endo K, Yuasa T, Ohno H, Hayashi K, Kakizaki M, et al.Galvanic corrosion behavior of orthodontic archwire alloyes coupled to bracket alloys. Angle Orthod 2006; 76:705-11.
- Bakhtari A, Bradley TG, Lobb WK, Berzins DW. Galvanic corrosion between various combinations of orthodontic brackets and archwires. Am J Orthod Dentofacial Orthop 2011; 140:25-31
CrossRef - Wataha JC. Biocompatibility of dental casting alloys: a review. J prosthet Dent 2000; 83:233-34.
CrossRef - Shin JS, Oh KH, Hwang CJ. In vitro surface corrosion of stainless steel and niti orthodontic appliances.Aust orthod J 2003;19:13-8
- Bishara SE, Barrett RD, Selim MI. Biodegradation of orthodontic appliances. Part II. changes in the blood level of nickel. Am J Orthod Dentofacial Orthop 1993; 103:115-9.
CrossRef - Faccioni F, Franceschetti P, Cerpelloni M, Fracasso ME. In vivo study on metal release from fixed orthodontic appliances and DNA damage in oral mucosa cells. Am J Orthod Dentofacial Orthop 2003; 124:687-93
CrossRef - Hwang CJ, Shin JS, Cha JY. Metal release from simulated fixed orthodontic appliances. Am J Orthod Dentofacial Orthop 2001; 120:383-91
CrossRef - Sfondirini MF, Cacciafesta V, Maffia E, Massironi S, Scribante A,Alberti G, et al. Chromium release from new stainless steel, recycled and nickel-free orthodontic brackets. Angle Orthod 2009; 79:361-7.
CrossRef - Singh DP, Sehgal V, Pradhan KL, Chandna A, Gupta R. Estimation of nickel and chromium in saliva of patients with fix orthodontic appliances. World J Orthod 2008;9:196-202.
- Retamoso LB, Luz TB, Marinowic DR, Machado DC, De Menezes LM, Freitas MPM et al. Cytotoxicity of sthetic, metallic, and nickel-free orthodontic brackets: cellular behavior and viability. Am J Orthod Dentofacial Orthop 2012; 142:70-4.
CrossRef - Westphalen GH, Menezes LM, Prá D, Garcia GG, Schmitt VM, Henriques JA et al. in vivo determination of genotoxicity induced by metals from orthodontic appliances using micronucleous and comet assay. Genet Mol Res 2008; 7:1259-93.
CrossRef - Natarajan M, Padmanabhan S, Chitaranjan A, Narasimhan M.Evaluation of the genotoxic effects of fixed appliances on oral mocusal cells and the relathonship to nickel and chromium concentrations An in-vivo study. Am J Orthod Dentofacial Orthop 2011;140:383-8
CrossRef - Angelieri F, Marconde JP, de Almeida DC, Salvadori DM, Ribeiro DA. Am J Orthod Dentofacial Orthop 2011; 139:504-9 .
CrossRef - Sarkar NK, Redmond W, Schwaninger B, Goldberg AJ.The chloride corrosion behavior of four orthodontic wires. J oral Rehabil 1983; 10:121-8.
CrossRef - Yonekura Y, Endo K, Lijima M, Ohno H, Mizoguchi I. In vitro corrosion characteristics of commercially available orthodontic wires. Dent Mater J 2004; 23:197-202.
CrossRef - Eliades T, Athanasiou AE. In vivo aging of orthodontic alloys: implications for corrosion potential, nickel release, and biocompatibility.Angle Orthod 2002; 72:222-37
- Kocadereli L, Atac PA, Kale PS, Ozer D. Salivary nickel and chromium in patients with fixed orthodontic appliances. Angle orthod 2000; 70:431-4.
- Ağaoğlu G, Arun T, Izgu B, Yarat A. Nickel and chromium levels in the saliva and serum of patients with fix orthodontic appliances. Angle orthod 2001; 71:375-9.
- Barret R, Bishara S, Quinne J. Biodegradation of orthodontic appliances. Part 1. Biodegradation of nickel and chromium in vitro.changes in the blood level of nickel. Am J Orthod Dentofacial Orthop 1993; 103:8-14.
CrossRef - Sahoo N,Kialasam V, Padmanabhan S, Chitharanjan AB. In-vivo evaluation of salivary nickel and chromium levels in conventional and self-ligating brackets. Am J Orthod Dentofacial Orthop 2011; 140:340-5.
CrossRef - Grimsdottir MR, Gjerdet NR, Hensten-Pettersen A. Composition and in vitro corrosion of orthodontic appliances. Am J Orthod Dentofacial Orthop 1992;101:525-32.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





