How to Cite | Publication History | PlumX Article Matrix
Effects of Dexmedetomidine on Surgical Stress Responses at Patients under CABG
Bahram Fariborz Farsad1, Maryam Janipour2*, Ziya Totonchi3, Farhad Gorjipour3 and Samira Oroji Omid3
1Pharm.D, BCPS, Assistant Professor, Rajaie Cardiovascular Medical and Research Center, Iran University of Medical sciences, Tehran, Iran.
2Department of Clinical Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University, Tehran, Iran.
3Rajaie Cardiovascular Medical and Research Center, Iran University of Medical Sciences, Tehran, Iran.
Corresponding Author E-mail: m.janipour92@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2296
ABSTRACT: Cardiopulmonary bypass (CPB) surgery with extracorporeal circulation produce changes in the immune system and plasma levels of inflammatory cytokines. we hypothesize that Dexmedetomidine as an adjuvant , modulates the inflammatory response after CABG. In a prospective, randomized, blind study, 31 patients were assigned to Dexmedetomidine (Dex) group and compared with control group of 30 patients. Dex was administered at a loading dose of 0.5 µg/kg for 10 min , followed by a continuous infusion of 0.5 µg/kg per hour until the completion of CABG with CPB . The endpoints used to assess inflammatory responses to mini – CPB were plasma tumor necrosis factor (TNF) – α , interleukin (IL – 6 ) and interleukin ( IL – 10) levels. The inflammatory markers (IL – 6 , IL – 10 , TNF – α ) were determined after Dex administration , before CPB and 24 hours after admission to ICU. Biochemical factors including glucose , creatinine , lactate , BUN, AST , ALT , LDH were determined before CPB, immediately after entering the ICU , 24 hr , 48 hr and 72 hr post admission to ICU. Hemodynamic variables were also determined. Dex group was associated with a significant reduction in urea and creatinine. There were no significant differences in glucose, lactate, liver enzymes, LDH , IL – 6, IL – 10 and hemodynamic variables. In contrast, the surgery – induced increase in TNF – α levels in the Dex group was significantly higher compared with the control group.
KEYWORDS: Coronary Artery Bypass grafting , Cardiopulmonary Bypass.
Download this article as:| Copy the following to cite this article: Farsad B. F, Janipour M, Totonchi Z, Gorjipour F, Omid S. O. Effects of Dexmedetomidine on Surgical Stress Responses at Patients under CABG. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Farsad B. F, Janipour M, Totonchi Z, Gorjipour F, Omid S. O. Effects of Dexmedetomidine on Surgical Stress Responses at Patients under CABG. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15962 |
Introduction
Dexmedetomidine (Dex) is a highly selective α2-adrenergic receptor agonist, that is used wildly as a sedative and analgesic agent in the ICU or as an anesthetic adjuvant (1). Dex preserves hemodynamic stability during cardiopulmonary bypass (CPB) surgery (2). In addition, Dex attenuates the stress responses caused by intubation and surgery (3,4). Due to its sympatholytic property Dex can decrease the myocardial oxygen consumption and heart rate (HR). Major Surgeries produce many complex changes as well as metabolic, endocrine, and immune complications called “stress response,” leading to a prolonged hospitalization. Due to this systemic response, many postoperative complications such as respiratory and coagulation disorders, wound infection, myocardial and neurologic dysfunction, changes in liver and kidney function with an increased mortality may occur (5 – 7). Complex interrelated pathways including generation or activation of cytokines, complement, neutrophils, mast cells, thrombin and other involved inflammatory mediators, produce inflammatory responses in CABG patients. Complex pathophysiological changes in SIRS can be compared to inflammatory responses at CPB and remain unclear (8).
Several interconnected mechanisms may have a role in the pathological alterations associated with CPB, such as blood contact with non- physiologic surfaces, body temperature fluctuations, anesthesia, surgical trauma, increased transmission of bacterial endotoxins across intestinal membrane and IR (ischemia/reperfusion) – induced injury (9). It leads to a systemic immunologic response with the release of arachidonic acid metabolites, endothelins, pro inflammatory cytokines, platelet-activating factors, leukocyte and endothelial adhesion molecules into circulation, causing over production of reactive oxygen species (10,11). Consequently, it is valuable to investigate the possible benefits of privileged anesthetic agents with anti-inflammatory capability. Anesthetic drugs can modulate stress responses and reduce CABG – induced morbidity and mortality. Dexmedetomidine, is a potential agent that little researches has been conducted to clarify its advantages (12). Dexmedetomidine exert sedative and anxiolytic properties without respiratory depression. It has analgesic effect known as “ opioid – sparing “ effect, and is able to reduce sympathetic discharge of central nervous system in a dose-dependent manner. There is certain evidences that Dexmedetomidine can protect sensitive organs, like heart, kidney and brain against IR injury (13). However, little knowledge is available about the exact molecular and cellular mechanism(s) playing role in dexmedetomidine protective effects. In this paper, systemic anti – inflammatory properties and possible cardioprotective effects of Dexmedetomidine in CABG, will be discussed.
Materials and Methods
61 patients undergoing on – pump CABG, were scheduled to take part in the study. Patients who had been divided randomly into two groups of Dex and control, treated by Dexmedetomidine (Hospira, Inc, Lake Forest, Il 60045 USA) and normal saline, respectively. Patients who had systemic inflammatory diseases, previous heart surgery, deep hypothermia, myocardial infarction within a month before surgery, unstable angina and severely left ventricular dysfunction (ejection fraction < 40%) were excluded from study. The study was confirmed by the Ethics Committee of the pharmacy school, Islamic Azad University. Informed consent forms was obtained from all of the patients before involving in the study. The patients’ systolic and diastolic blood pressure, heart rate (HR) and central venous pressure (CVP) values, were continuously measured and recorded. The blood pressure, CVP and HR were recorded at the following times: baseline (before administration of Dexmedetomidine or normal saline) (T1), after separation from pump (T2), after entering the ICU (T3), 6 h after entering the ICU (T4), 12h after entering the ICU (T5) , 24h after entering the ICU (T6), 36h after entering the ICU (T7), 48h after entering the ICU (T8). In the operation room, a pulse oximetery and ECG leads were continuously monitored. To measure systemic arterial blood pressure, A 20 G radial artery catheter was inserted. A double cavity central venous catheter was placed into the right internal jugular vein before induction of anaesthesia. Anesthesia was induced with 10 – 15 µg/kg fentanyl and 0.1 mg/kg midazolam. A bolus dose of 0.1 mg/kg pavulon was given at 45 min intervals to facilitate endotracheal intubation. Anesthesia was maintained by 5 µg/kg/hr fentanyl and 1 µg/kg/min midazolam after tracheal intubation. The hypotension, was corrected by Phenylephrine infusion, when MAP or systolic pressure decreased below 40 and 80 mmHg, respectively. Dex was infused at a loading dose of 0.5 µg/kg for 10 min, then Dex infusion was maintained at a rate of of 0.5 µg/kg/hr until the completion of the surgery.
Patients were administered Dex pump infusion at a loading dose of 0.5 µg/kg for 10 min, followed by a continuous pump infusion of 0.5 µg/kg/hr until the completion of the surgery. At the same time, the control group, recieved same infusion of normal saline. Surgery was started through a median sternotomy in all of the patients. The left internal mammary artery (with pedicle) and the greater saphenous vein were harvested. CPB was conducted using a roller pump and membrane oxygenator. All patients received 10 ml/kg of Ringer solution before surgery. Deep hypothermia was induced and the body temperature was maintained at 30 – 32 °C. Pharmacological support was given in accordance with the patients’ hemodynamic necessities. The surgeons at the operating room and the nurses at ICU department were blind to treated regimens.
Central venous blood samples were taken at times mentioned before. For detection of plasma levels of inflammatory markers, Blood samples were centrifuged at 3000 rpm for 10 min, then plasma were isolated and kept at nitrogen tank, there the samples were stored at a temperature of 196 °C below zero until termination of study. The levels of TNF- alpha, IL-6 and IL-10 were measured by chemical analysis (commercial kits: Bio Orbit, UK) by enzyme-linked immunosorbent assay (ELISA). All assays were performed on the basis of the manufacturers’ instructions. In order to measure other factors, blood samples were directly transferred to laboratory and were analyzed by (Auto Analyser Hitachi 917 and 912 RA 1000). Cross – clamping time, CPB duration and the number of grafted vessels were also recorded.
Statistical analysis
Statistical analyses were implemented using stata version 13.0 software program. Individual data points were considered to be irrelevent if >2 SD from the mean. Data is s as mean ± SD. Difference in groups were considered significant if P <0.05. Data was analysed using Reapeted Measured ANOVA and T test. Statistical significance in differences between the groups was presumed when P <0.05.
Table 1 : Characteristics of Patients
| parameters | Dex group
(n = 31) |
control group
(n = 30) |
P value |
| Age (years)
Weight (kg) BMI (kg m-2) LV ejection fraction (%) Comorbidities HLP , n(%) HTN , n(%) Addiction, n(%) Liver diseases, n(%) Kidney diseases, n(%) Diabetes, n(%)
|
58.4 (10.1)
72.8 (21.3) 1.84 (0.28) 43 (9.45)
7 (22.58) 15 (48.39) 15 (48.39) 0 2 (6.45) 8 (25.8) |
61.2 (11.7)
72.6 (15.6) 1.81 (0.24) 44.83 (12.41)
7 (23.33) 19 (65.52) 7 (23.33) 0 1 (3.33) 17 (56.67) |
0.33
0.97 0.66 0.51
0.994 0.181 0.042 – 0.573 0.014 |
Data are shown as incidence (%) or mean ± (SD). BMI, body mass index; EF, ejection fraction, HTN, hypertension , HLP, hyperlipidemia
Table 2: Comparison of Surgical Parameters
| parameters | Dex group | Control group | P value |
| Cross-clamping time (min)
Duration of CPB (min) Number of grafted vessels Need for Inotropic support , n (%) Milrinone EPI NEPI Blood transfusion PLT (unit) FFP (unit) PC (milliliter)
|
46.22 (19.9)
86.9 (29.1) 3.53 (0.83)
0 6(19.35) 1(3.23)
0.45(0.88) 0.45(0.99) 104.8(168) |
45.03 (22.6)
88.8 (42.8) 3.53 (0.73)
1(3.33) 8(27.59) 0
0.63(1.03) 0.63(1.21) 133.3(204) |
0.53
0.89 0.99
0.305 0.451 0.329
0.48 0.51 0.60 |
Values are provided as incidence (%) or mean ± (SD). EPI: epinephrine, NEPI: norepinephrine, PLT: platelet, FFP: fresh frozen plasma, PC: packed cell.
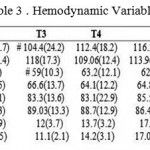 |
Table 3: Hemodynamic Variables
|
Data are presented as the mean ± (SD). # P<0.05 as compared to the control group; MAP, mean blood pressure; HR, heart rate, CVP, central venous pressure.
Result
To study the effects of Dexmedetomidine on the surgical stress responses, first we ignore any confounding data including age, sex, weights and comorbidities. However in addiction and diabetes there were significant differences between two groups . Patients undergoing CABG with CPB were randomly allocated into two groups, receiving either Dex (n=31) or normal saline (n=30). The obtained information about demographic Characteristics of patients, showed no significant differences between two groups (Table 1).
The duration of cross-clamping and CPB, grafted vessel numbers, inotropic support and need for blood transfusion showed no significant variation between two groups (Table 2).
After separation from pump (T2) and entering the ICU (T3) Dex received patients had lower SBP (102.03 ± 17.7 at T2 and 104.4 ± 24.2 at T3 respectively), compared to control group (114.06 ± 16.4 at T2 and 118 ± 17.3 at T3) (Table 3). In addition, Dex group patients also had a lower DBP at T3 when compared control group (59 ± 10.3 at T3 in Dex group and 66.6 ± 13.7 in control group) (Table 3).
Blood samples were collected from all patients in both groups and BUN and Creatinine levels were determined. patients at the two treated groups had an overall increase in plasma BUN level after entering the ICU (P<0.01) (Figure 2A). However, in comparion with control group, Dex group patients clearly showed decrease in BUN at 72 hr after entering the ICU (P< 0.01). Creatinine levels at 24 hr after entering the ICU showed significant
decrease between two groups and it was lower in Dex treated patients (p<0.05) , the differences at Creatinine level between two groups went to be nearly significant (Figure 2B). Meanwhile, patients in both group showed no differences at other biochemical endpoints including AST, ALT, LDH and lactate. (Figure 2C and Figure 3).
Plasma IL-6 (Figure 1B) levels increased after CPB compared with baseline. but this increase was clearly higher in the control group compared with the Dex group. Plasma TNF-α levels (Figure 1A) were increased after surgery compared with baseline. However, the increase in TNF-a levels was significantly higher in the Dex group compared the control group (p<0.05) . Plasma IL-10 levels (Figure1C) were sharply increased in both groups during CABG surgery and decreased after CPB.
Dexmedetomidine did not modify the rate of decrease in IL-10 levels, the peak level in Dex group was slightly higher than control group, but no differences were existed between the two groups.
 |
Figure 1: Concentrations of pro inflammatory cytokines (TNF – α panel A , IL- 6 panel B) and anti inflammatory cytokine (IL – 10 panel C) at baseline , after completion of CPB, 24 hr after entering the ICU. Data are represented as mean (SD). #P < 0.05 vs control.
|
 |
Figure 2: Biomarkers of , kidney and myocardial injuries at various time points in patients undergoing CPB. Data are represented as mean (SD). #P < 0.05 vs control .
|
 |
Figure 3: Bio markers of liver and myocard muscle injuries . Data are represented as mean (SD). #P< 0.05 vs control.
|
Discussion
The main purpose of the present study is to evaluate the stress response in patients under CABG with Extracorporeal circulation. The use of specific anesthetic as anti- inflammatory agents, could attenuate these surgical inflammatory responses and helps to reduce complications at postoperative recovery period. Activation of the central α2-adrenergic receptors, leads to reduction in hemodynamic variables, such as blood pressure and HR, and consequently modulate the surgical stress responses. Our study indicates some of the clinical significance of Dex administration (12).
In this study, reduction in hemodynamic values including the HR, blood pressure and CVP was observed at Dex group patients, although it was not significant. Inhibition of central sympathetic discharge, attenuation of the peripheral sympathetic tone and stimulation of vagus nerve, results in a reduction of HR and MAP, leading to decline in cardiac muscle oxygen demand and heart afterload, providing heart protection, particularly in patients suffering from coronary artery stenosis (14). Laryngoscopy and endotracheal intubation stimulate remarkable neuro- endocrine responses that increase the risk of perioperative myocardial ischemia and infarction. The perioperative administration of dexmedetomidine may attenuate stress responses and helps to preserve endocardial perfusion (4). Dexmedetomidine as sympatholytic anesthetic adjuvant, stables hemodynamic fluctuations during surgery and recovery period, modifies stress induced sympatho-adrenal reactions to intubation, therefore reveals better outcome (15).
In this study glucose and lactate values showed no significant differences between two groups of patients. There is no obvious information about Dex inhibitory effects on cortisol synthesis in humans at short – term studies (16 – 18).
In fact, Bekker et al. (19) assumed that intraoperative DEX infusion adjust the severe post – operative increase in plasma cortisol levels by reducing stress responses and improvement of quality of recovery in patients undergoing major spinal surgery. However, we did not measure cortisol levels between the two groups of patients.
Venn et al. (18) conducted a research to evaluate the endocrinal, cardiovascular and anti – inflammatory effects of Dex in cardiac surgery patients needing ICU sedation.
They concluded that supra therapeutic concentrations of dexmedetomidine can inhibit cortisol synthesis and decrease surgical stress responses. Mukhtar et al. (20) understood that dexmedetomidine alleviate hyperglycemic reactions to surgery significantly compared to control group, indicating sympathoadrenal response suppression.
Tissue ischemia – reperfusion (IR) during CPB, activates innate immune system and cytokine cascade, leads to inflammatory response. This response is the primary cause of IR injury, and could be more harmful than the original surgical incisions (21, 22). Dexmedetomidine may present its Anti – inflammatory effects through provoking vagus nerve and stimulation of cholinergic anti – inflammatory pathways (23).
Many reports have been published about α2 – adrenergic receptor agonists effects on cytokine and TNF – α production (24, 25).
Taniguchi and colleagues (26) proved that Dex prevent cytokines to response against endotoxemia. One of the possible anti – inflammatory mechanisims of Dexmedetomidine is to modulate cytokine production by macrophages and monocytes. Hofer et al, found that Dex infusion in Sepsis, can diminish cytokine production (27). They found that preoperative Dex or clonidine administration in sepsis – induced rat models, reduces proinflammatory cytokines such as IL – 1, IL-6 and TNF – α and finally improves survival rate. Therefore, they recommended that Dex administration before surgery, would be a good option in seriously – ill patients undergoing CABG (28).
Some of these findings are in accordance with our results but interestingly in our study , TNF values in Dex group patients are significantly higher than control group , This result is in contrast to previous studies and we do not have a clear reason to justify it. It is suggested that more detailed studies be conducted in order to decode the subject in this regard.
In current study we assess BUN and Creatinine levels as indicators of renal function . As yet, no definite strategy for preventing AKI after cardiac surgery exists. Various factors including ischemia-reperfusion injury, renal hypoperfusion, systemic inflammation, and embolic events, especially in association with cardiopulmonary bypass (CPB), have been thought to be responsible for the development of AKI after cardiac surgery (29-31). Hemodynamic stability, sympathetic activity and renal function have a close relation. Surgical stress-induced sympathetic stimulation increases catecholamine release, resulting in hemodynamic instability vasoconstriction of renal arteries, which is noxious to renal function (30). Consequently, Dex sympatholytic action through α-2 adreno-receptors activation, is helpful hemodynamic stability and renal IR injury reduction (32 – 34).
Activation of α-2 adrenoreceptors located on renal vasculature and tubules, prevents renin secretion, increases glomerular filtration and salt and water excretion (35).
Post – CABG Renal dysfunction increases serum creatinine, leads to increased mortality and morbidity, prolonged hospitalization, and more medical costs (36). Dex mechanism of action on renal function are complex. Alpha- 2 agonists show a diuretic effect with increased salt and water excretion (37). Several animal studies, demonstrated possible beneficial effects of Dexmedetomidine on renal function.
First, dexmedetomidine stimulate presynaptic α2 receptors, therefore inhibits the release of sympathetic neurotransmitters (epinephrine and norepinephrine) into synaptic space (38,39). Attenuation of sympathicoadrenal hyperactivity and hemodynamic variations, results in better preservation of renal function. Second, Dexmedetomidine activates endothelial a2 adrenoreceptor, which are exist widely in renal peritubular vesseles and tubules and produces nitric oxide-induced vaso relaxation (40).
Conclusion
In this study, we demonstrated that DEX could attenuate the increase in IL-6, BUN, serum creatinine levels and stables hemodynamic variables. According to this findings Dex has good effect on kidney function at patients under CABG surgery with mini-CPB. Despite of our expectations TNF-α values were significantly higher in Dex group. One of our limitations in this study was the small number of enrolled patients. So, large-scale clinical trials are required to further investigate anti-inflammatory effects of Dex during major surgeries.
References
- Afonso J and Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol 2012; 62: 118-133.
CrossRef - Kabukcu HK, Sahin N, Temel Y and Titiz TA. Hemodynamics in coronary artery bypass surgery: effects of intraoperative dexmedetomidine administration. Anaesthesist 2011; 60: 427-431.
CrossRef - Menda F, Koner O, Sayin M, Ture H, Imer P and Aykac B. Dexmedetomidine as an adjunct to anesthetic induction to attenuate hemodynamic response to endotracheal intubation in patients undergoing fast-track CABG. Ann Card Anaesth 2010; 13: 16-21.
CrossRef - Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H and Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth 2012; 15: 39-43.
CrossRef - M. Sander, C. von Heymann, V. von Dossow et al., “Increased interleukin-6 after cardiac surgery predicts infection,” Anesthesia and Analgesia, vol. 102, no. 6, pp. 1623–1629, 2006.
CrossRef - J. M. Murkin, “Panvascular inflammation and mechanisms of injury in perioperative CNS outcomes,” Seminars in Cardiothoracic and Vascular Anesthesia, vol. 14, no. 3, pp. 190–195, 2010.
CrossRef - M. E. Plomondon, J. C. Cleveland Jr., S. T. Ludwig et al., “Off- pump coronary artery bypass is associated with improved risk adjusted outcomes,” Annals of Thoracic Surgery, vol.72, no.1, pp. 114–119, 2001.
CrossRef - S.Wan,J.-L.Leclerc,andJ.-L.Vincent, “Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies,” Chest, vol. 112, no. 3, pp. 676–692, 1997.
CrossRef - M. M. Elahi, J. S. Khan, and B. M. Matata, “Deleterious effects of cardiopulmonary bypass in coronary artery surgery and scientific interpretation of off-pump’s logic,” Acute Cardiac Care, vol. 8, no. 4, pp. 196–209, 2006.
CrossRef - B. M. Matata, A. W. Sosnowski, and M. Gali˜nanes, “Off-pump bypass graft operation significantly reduces oxidative stress and inflammation,” Annals of Thoracic Surgery, vol. 69, no. 3, pp. 785–791, 2000.
CrossRef - B. M. Matata and M. Gali ˜ nanes, “Cardiopulmonary bypass exacerbates oxidative stress but does not increase pro inflammatory cytokine release in patients with diabetes compared with patients without diabetes: regulatory effects of exogenous nitric oxide,” Journal of Thoracic and Cardiovascular Surgery, vol. 120, no. 1, pp. 1–11, 2000.
CrossRef - Bulow NMH, Colpo E, Duarte MF, Correa EFM, Schlosser RS, Lauda A, et al. Inflammatory response in patients under coronary artery bypass grafting surgery and clinical implications: a review of the relevance of dexmedetomidine use. ISRN Anesthesiology 2014, doi: 10.1155/2014/905238.
CrossRef - J. E. Hall, T. D. Uhrich, J. A. Barney, S. R. Arain, and T. J. Ebert, “Sedative, amnestic, and analgesic properties of small- dose dexmedetomidine infusions,” Anesthesia and Analgesia, vol. 90, no. 3, pp. 699–705, 2000.
CrossRef - H. M. Oudemans-van Straaten, P. G. M. Jansen, F. J. Hoek et al., “Intestinal permeability, circulating endotoxin, and postoperative systemic responses in cardiac surgery patients,” Journal of Cardiothoracic and Vascular Anesthesia, vol. 10, no. 2, pp. 187– 194, 1996.
CrossRef - B. Scheinin, L. Lindgren, T. Randell, H. Scheinin, and M. Scheinin, “Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl,” British Journal of Anaesthesia, vol. 68, no. 2, pp. 126–131, 1992.
CrossRef - Maze M, Virtanen R, Daunt D, Banks SJ, Stover EP, Feldman D. Effects of dexmedetomidine, a novel imidazole sedative-anesthetic agent, on adrenal steroidogenesis: in vivo and in vitro studies. Anesth Analg 1991; 73: 204–208.
CrossRef - Venn RM, Bryant A, Hall GM, Grounds RM. Effects of dexmedetomidine on adrenocortical function, and the cardio- vascular, endocrine and inflammatory responses in post- operative patients needing sedation in the intensive care unit. Br J Anaesth 2001; 86: 650–656, doi: 10.1093/bja/86.5.650.
CrossRef - Bulow NM, Barbosa NV, Rocha JB. Opioid consumption in total intravenous anesthesia is reduced with dexmedetomidine: a comparative study with remifentanil in gynecologic video laparoscopic surgery. J Clin Anesth 2007; 19: 280– 285, doi: 10.1016/j.jclinane.2007.01.004.
CrossRef - Bekker A, Haile M, Kline R, Didehvar S, Babu R, Martiniuk F, et al. The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol 2013; 25: 16–24,
doi: 10.1097/ ANA.0b013e31826318af.
CrossRef - Mukhtar AM, Obayah EM, Hassona AM. The use of dexmedetomidine in pediatric cardiac surgery. Anesth Analg 2006; 103:52–56.
CrossRef - Carden DL and Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 2000; 190: 255-266.
CrossRef - Medzhitov R. Origin and physiological roles of inflammation. Nature 2008; 454: 428-435.
CrossRef - Xiang H, Hu B, Li Z and Li J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation 2014; 37: 1763-1770.
CrossRef - R.H.Straub,M.Herrmann,G.Berkmilleretal.,“Neuronal regulation of interleukin 6 secretion in murine spleen: adrenergic and opioidergic control,” Journal of Neurochemistry, vol. 68, no. 4, pp. 1633–1639, 1997.
CrossRef - J. Szel´enyi, J. P. Kiss, and E. S. Vizi, “Differential involvement of sympathetic nervous system and immune system in the modulation of TNF-𝛼 production by 𝛼2- and 𝛽-adrenoceptors in mice,” Journal of Neuroimmunology,vol.103,no.1,pp.34–40, 2000.
CrossRef - T. Taniguchi, Y. Kidani, H. Kanakura, Y. Takemoto, and K. Yamamoto, “Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats,” Critical Care Medicine, vol. 32, no. 6, pp. 1322–1326, 2004.
CrossRef - S. Hofer, J. Steppan, T. Wagner et al., “Central sympatholytics prolong survival in experimental sepsis,” Critical Care, vol. 13, no. 1, article R11, 2009.
CrossRef - U. Koca, C¸. G. Olguner, B. U. Erg¨ur et al., “The effects of dexmedetomidine on secondary acute lung and kidney injuries in the rat model of intra-abdominal sepsis,” The Scientific World Journal, vol. 2013, Article ID 292687, 11 pages, 2013.
- Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32.
CrossRef - Rosner MH, Portilla D, Okusa MD. Cardiac surgery as a cause of acute kidney injury: pathogenesis and potential therapies. J Intensive Care Med. 2008;23:3–18.
CrossRef - Calvert S, Shaw A. Perioperative acute kidney injury. Perioper Med (Lond). 2012;1:6.
CrossRef - Myles PS, Hunt JO, Holdgaard HO, et al. Clonidine and cardiac surgery: haemodynamic and metabolic effects, myocardial ischaemia and recovery. Anaesth Intensive Care. 1999;27:137–147.
- Kulka PJ, Tryba M, Zenz M. Preoperative alpha2-adrenergic receptor agonists prevent the deterioration of renal function after cardiac surgery: results of a randomized, controlled trial. Crit Care Med. 1996;24:947–952.
CrossRef - Gu J, Sun P, Zhao H, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15:R153.
CrossRef - Gellai M, Ruffolo RR Jr. Renal effects of selective alpha-1 and alpha-2 adrenoceptor agonists in conscious, normotensive rats. J Pharmacol Exp Ther. 1987;240:723–728.
- Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15: 1597–1605.
CrossRef - F. T. Billings IV, S. W. C. Chen, M. Kim et al,“ 2-Adrenergic agonists protect against radio contrast-induced nephropathy in mice,” American Journal of Physiology—Renal Physiology, vol. 295, no. 3, pp. F741–F748, 2008.
CrossRef - Xu H, Aibiki M, Seki K, et al. Effects of dexmedetomidine, an alpha2- adrenoceptor agonist, on renal sympathetic nerve activity, blood pressure, heart rate and central venous pressure in urethane- anesthetized rabbits. J Auton Nerv Syst. 1998;71:48–54.
CrossRef - Ebert TJ, Hall JE, Barney JA, et al. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93: 382–394.
CrossRef - Taoda M, Adachi YU, Uchihashi Y, et al. Effect of dexmedetomidine on the release of [3H]-noradrenaline from rat kidney cortex slices: characterization of alpha2-adrenoceptor. Neurochem Int. 2001;38: 317–322.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





