How to Cite | Publication History | PlumX Article Matrix
Anggi Shestita Wulandari, Olivia Mayasari Tandrasasmita, and Raymond Rubianto Tjandrawinata
Dexa Laboratories of Biomolecular Sciences, PT Dexa Medica, Industri Selatan V, Block PP No. 7, Jababeka Industrial Estate II, Cikarang, West Java, 17550, Indonesia.
Corresponding Author E-mail: raymond@dexa-medica.com
DOI : http://dx.doi.org/10.13005/bbra/2269
ABSTRACT: Lactobacillus fermentum DLBSA204 is an isolated lactic acid bacterium (LAB) from human breast milk. This study focused on the effects of L. fermentum DLBSA204 on macrophage cell proliferation with MTS assay using murine RAW 264.7 macrophage-like cell line as well as related molecular changes associated with macrophage activation. It was demonstrated that L. fermentum DLBSA204 can activate macrophage cell by increasing Nitric Oxide and cytokine expression level. Moreover, this LAB was able to increase phagocytic activity of macrophage. L. fermentum DLBSA204 was also able to inhibit the adhesion of Streptococcus pneumoniae on lung cells. In conclusion, L. fermentum DLBSA204 works as an immunomodulator and macrophage activator.
KEYWORDS: Lactobacillus fermentum; immunomodulator; macrophage; Streptococcus pneumoniae
Download this article as:| Copy the following to cite this article: Wulandari A. S, Tandrasasmita O. M, Tjandrawinata R. R. Immunomodulatory and Macrophage Activating Activity of Lactobacillus fermentum DLBSA204 in Response to Respiratory Infection in a Cellular Model. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Wulandari A. S, Tandrasasmita O. M, Tjandrawinata R. R. Immunomodulatory and Macrophage Activating Activity of Lactobacillus fermentum DLBSA204 in Response to Respiratory Infection in a Cellular Model. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15932 |
Introduction
Human breast milk has been known to protect infants from infections and diseases caused by various types of pathogenic bacteria. This protective property is due to the immunomodulatory compounds which are also a major factor in the initiation and development of neonatal gut microbiota (1, 2, 3). The human breast milk also contains potential probiotic that possess antimicrobial (4) and immunomodulatory property which may boost the infant’s immunity (5).
Probiotics are live microorganisms, which when consumed in an adequate dose, confer health benefits to its host. Members of the genera Lactobacillus is known to be one of the main constituents of normal microflora in the human body. They especially colonize human oral cavity, rectum (6), and breast milk (4, 7, 8) where they contribute profoundly to the health of its host and in the case of breast milk, to the infant. Some of these health benefits include prevention and treatment of common diseases in children related with respiratory tract infections (9, 10).
Respiratory tract is divided into 2 parts; the upper and lower respiratory tract. Infection of both upper and lower respiratory tract are caused by various viruses and bacteria, with the most common being Streptococcus pneumoniae. Treatments for these infections are drugs for symptomatic treatment, herbal remedies, zinc supplementation and antibiotic (11, 12). A clinical study with 326 eligible children has demonstrated probiotic treatment that consisted of Lactobacillus acidophillus NFM (N110) or L. acidophillus NCFM in combination with Bifidobacterium animalis subsp lactis Bi-07 (N112) for 6 months was able to help treat respiratory tract infection by reducing incidence and duration of fever, rhinorrhea and cough (9). Other probiotic, Lactobacillus rhamnosus GG has also been known to help treat respiratory tract infection by modulating host immune response (13). From these examples, it is evident that the ability to modulate the host’s immune system is critical to treatment of respiratory tract infection. The stimulation of immune system by probiotic bacteria is species and strain-dependent. Bifidobacterium and Lactobacilli, which are both probiotic bacteria, possess different mitogenic activity on splenocytes (14). Different strains of Lactobacilli also induce different cytokine expression in in vitro and in vivo study, such as TNF-α and IL-6 (15, 16). Therefore, efficacy of each specific strain has to be investigated.
In most studies, in vitro immunomodulatory activity was observed using bacterial cultures exposed to RAW 264.7 macrophage cells (17, 18). In this study, the biological activity of L. fermentum DLBSA204, a lactic acid bacteria (LAB) strain isolated from human breast milk, was evaluated in RAW 264.7 and A549 cells. This LAB was evaluated for its potential in promoting macrophage activation, competition for adhesion with S. pneumonia, and antioxidant activity in vitro.
Materials and Methods
Lactobacillus fermentum DLBSA204 was isolated in our laboratory from human breast milk. De Man, Rogosa and Sharpe broth (MRSB); De Man, Rogosa and Sharpe agar (MRSA), trypticase soy broth (TSB) and trypticase soy agar (TSA) were purchased from © Merck KGaA, Darmstadt, Germany. RAW 264.7 (ATCC® TIB71™), A549 (ATCC® CCL185™) and Streptococcus pneumonia (ATCC® 49619™) were derived from ATCC, Manassas, USA. Dulbecco’s Modified Eagle’s Medium (DMEM), F-12K medium, L-glutamine, Fetal Bovine Serum (FBS) and penicillin/streptomycin (P/S) were acquired from Gibco® (Thermo-fischer scientific, Waltham, MA, USA). TRizol® was obtained from Invitrogen (Thermo-fischer scientific, California, United States), while PGE2 ELISA kit was obtained from GE Healthcare (Little Chalfont, United Kingdom). One Step RNA PCR Kit RT, RNasin, dNTP mix, oligo dT, MgCl2, Taq polymerase, GoTaq Green master mix, Track It DNA Ladder 100 bp, and CellTiter 96® Aqueous one solution cell proliferation assay (MTS) were purchased from Promega (Madison, USA). Lipopolysaccharide (LPS) from Escherichia coli 055:B5, DPPH (2,2-diphenyl-1-picylhydrazyl), Griess reagent and all other chemicals used in this study were obtained from Sigma-Aldrich (Saint Louis, USA) and were of analytical grade.
Preparation of LAB isolate
fermentum DLBSA204 was isolated from human breast milk and has been identified with API system that was based on carbohydrate fermentation profile. The identification was done using the API 50 CHL kit (BioMérieux; USA). The result proved that the strain is Lactobacillus fermentum. This strain was further cultured at 37°C in MRSB for 18-20 hours. The resulting pellet was collected by centrifugation at 4000xg for 10 minutes using Hettich Mikro 200 centrifuge (Hettich, Massachusetts, United States), and then washed twice with PBS 1x (137 mM NaCl, 2.7 mM Kcl, 10 mM Na2HPO4, 2 mM KH2PO4). For the experiment, 1×109 colony forming unit (CFU) of bacteria per mL was added to the cell culture. Untreated cells were used as control, while LPS 250 ng/ml was used as positive control to induce inflammation. S. pneumonia was cultured in trypticase soy broth (TSB) for 20 hours and was used in adhesion and phagocytic activity assays.
Cell culture
Mouse macrophage cell line RAW 264.7 was cultured in DMEM supplemented with 10% (v/v) FBS and 1% P/S 100 U/ml at 37°C in a humidified incubator with 5% CO2 (New Brunswick Scientific Co. Inc, New Jersey, United States) as described previously (19). Human lung cancer cell line A549 was grown in F12K medium supplemented with 10% (v/v) FBS, and 1% P/S (100 U/ml) at 37°C in a 5% CO2 humidified incubator.
Macrophage cell viability assay
MTS assay was used to evaluate L.fermentum DLBSA204 as macrophage activator. RAW 264.7 cells were seeded onto 96-well plate. As much as 109 CFU/ml of DLBS L.fermentum DLBSA204 was added to the culture and incubated for 24h. MTS solution 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (0.5mg mL-1) was added to the cells for 2h to induce reaction. Absorbance at 490 nm was measured with model 680 microplate reader (BioRad Laboratories, CA, USA).
Effect of L. fermentum DLBSA204 on NO production
RAW 264.7 cells were seeded in a six-well plate and incubated at 37°C with 5% CO2. As much as 109 CFU/ml of L.fermentum DLBSA204 was added to the culture and incubated for 24h at 37°C. NO in culture supernatant was measured as concentration of nitrite using Griess reagent. One hundred microliter of cultured supernatant was added into the 96-well plate. Then 100 μl of Griess reagent was added to each well and allowed to stand for 15 min at 25°C. The absorbance at 540 nm was measured with model 680 microplate reader (680, BioRad Laboratories, CA, USA).
Phagocytic activity of RAW 264.7 cells
A ciprofloxacin protection assay was used to quantify the ability of RAW 264.7 cells to ingest S. pneumonia. A 24-well plate was inoculated with RAW 264.7 cells suspended in DMEM with density of 25 x 106 cell/ml and incubated at 370C with 5% CO2 in a humidified incubator. The cells were treated with 250 ng/ml LPS and L. fermentum DLBSA204 109 CFU/ml for 24 hours. After incubation, 108 CFU/ml of S. pneumonia was added to each sample for 15 minutes. Fifteen minutes is the optimal incubation time for macrophage to phagocyte S. pnuemoniae. Following incubation, the medium was discarded and the cells were incubated for 2 hours in the presence of 1 ml DMEM supplemented with 25 μg/ml of ciprofloxacin. Upon completion of incubation, all cells were washed thoroughly with PBS 1x to remove all traces of antibiotic, prior to addition of RIPA buffer (50 mM Tris pH 6.8, 0.5% (wt/vol) sodium dodecyl sulfate, 150 mM sodium chloride, 1% NP-40, 2 mM ethylenediaminatetraacetic acid (EDTA) and 1x protease inhibitor). Recovered S.pneumoniae from lysed RAW 264.7 cells were serially diluted and plated on tryptic soy agar (TSA) supplemented with sheep blood. Enumeration of recovered bacteria was done by colony count.
Gene expression analysis
TRIzol® reagent (Thermo Fisher Scientific) was used to homogenize cells and isolate total RNA. cDNA synthesis was performed using a reverse transcription system kit according to the manufacturer’s instructions. Polymerase chain reaction (PCR) was performed to amplify a specific gene using oligonucleotide primer designed by Primer 3 software as described previously (20, 21). The gene sequences were obtained from the Genebank database. Genes involved in macrophage activation (primer sequences are shown in table 1) were detected and amplified in a mixture of 12.5μL Go Taq Green master mix, a pair of target genes with a final concentration of 1 μM, a pair of internal controls with a final concentration of 0.2 μM, 3μL cDNA, and nuclease-free water to a total of 25μL. PCR process generally consisted of initial denaturation at 940C for 3 minutes; 30 cycles of denaturation at 940C for 30 seconds, primer annealing at 60-610C for 30 seconds, and elongation at 720C for 1 minute. An additional elongation was performed at 720C for 5 minutes. Reverse transcription polymerase chain reaction (RT-PCR) was performed using PCR Biometra. The targeted gene expression was quantified using Quantity One software and the arbitrary unit was measured by comparing the expression of target gene with internal control expression.
Table 1: Primer Sequences
| Primer | Forward sequence | Reverse sequence |
| TNF-α | AAC TTC GGG GTG ATC GGT CC | CAA ATC GGC TGA CGG TGT GGG |
| IL-6 | CCG GAG AGG AGA CTT CAC AG | ACA GTG CAT CAT CGT TGT TC |
| GAPDH | TCA CCA CCA TGG AGA AGG C | GCT AAG CAG TTG GTG GTG CA |
Measurement of Lysozyme Release in Culture media
Lysozyme release was observed from the lysis rate of Micrococcus lysodeikticus. RAW 264.7 cells were seeded in a six-well plate and treated with LPS 250 ng/ml and DLBSA204 109 CFU/ml. The cells were incubated for 24h at 370C in 5% CO2 in a humidified incubator. Cell supernatant was collected and centrifuged at 4000xg for 10 minutes. The supernatant was collected and mixed with 950 ml of M. lysodeikticus suspension of 200 mg.ml-1 in 0.05M sodium phosphate buffer (pH 6.2). The mixture of cell supernatant and M. lysodeikticus was incubated at 25ºC, and its optical density (OD) was measured at the 5th and 6th minute using a spectrophotometer with absorbance at 530 nm. The change in absorbance was compared to the standard solution of crystalline egg white lysozyme. One activity is equivalent to a decrease of 0.001 absorbance unit per minute.
Adhesion competition of L. fermentum DLBSA204 and S.pneumoniae with Scanning Electron Micrograph (SEM) and Total Plate Count
Observation of adhesion competition was analyzed microscopically using SEM and was counted by plating the attached bacteria in the appropriate medium. Prior to the observation with SEM, the A549 cells were grown on a cover slide. After 24 hours, the cells were treated with the L. fermentum DLBSA204 and S.pneumoniae, and incubated for another 24 hours. Then, the cell was washed with PBS buffer for several times and air-dried. The cells were post-fixed in 2% formalin for an hour, instead of glutaraldehyde. The cover slide was soaked in PBS buffer for 15 minutes and dehydrated through graded ethanol solutions. The cell was air-dried, coated with gold and examined with JEOL JSM 6510 scanning electron microscope (Peabody, MA, USA).
In total plate count method, the A549 human lung cells were seeded in a 12-well plate and incubated at 37°C with 5% CO2. L. fermentum DLBSA204 was added to the culture and incubated at 2h and 24h. Then the culture was washed 3 times with sterile PBS and split using trypsin EDTA. The number of viable attached-bacteria was quantified and expressed as CFU. The CFUs were determined by plating the diluted bacterial suspension on MRSB or TSB plates depending on the bacterial strains.
Scavenging activity of DPPH Radical
In this study, DPPH (2,2-diphenyl-1-picylhydrazyl) was used as a stable radical. Bacteria was inoculated to MRSB and incubated at 37oC for 18-20 hours. The pellet of bacteria was collected and resuspended in PBS. One hundred microliter of the sample was added to 2 ml of DPPH in methanol, mixed vigorously and allowed to stand in the dark at room temperature for 30 minutes. The absorbance was measured at 517 nm using a spectrophotometer (Helios, Thermo Fisher Scientific, Waltham, MA USA). The blank standard was prepared by replacing the extract with methanol. Radical scavenging activity of the samples was expressed using the following equation:
DPPH radical scavenging activity (%) = [1- (OD517, sample/ OD517, blank)] x 100 (22)
Statistical Analysis
The statistical difference between the test and control samples was determined by Student’s t-test analysis using the Stat-View software package (Abacus Concepts, Piscataway, NJ, USA). Values are expressed as means ± standard deviation for at least two independent experiments.
Results
Effect of DLBSA204 on Macrophage Proliferation
Effect of L. fermentum DLBSA204 on the viability of RAW 264.7 macrophage cells was investigated. The proliferation of RAW 264.7 cells in the presence of L. fermentum DLBSA204 after 24h increased 21.42% compared to control group treated with medium only (fig.1), while proliferation of RAW 264.7 cell which was treated with LPS increased 34.6%. LPS is used as positive control because it can induce inflammation, which activates macrophage as immune system alert.
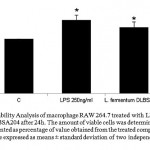 |
Figure 1: Cell Viability Analysis of macrophage RAW 264.7 treated with LPS 250 ng/ml and L. fermentum DLBSA204 after 24h. The amount of viable cells was determined by MTT assay. The result is presented as percentage of value obtained from the treated compared to untreated control. Values are expressed as means ± standard deviation of two independent experiments. Note: *P<0.05
|
Induction of NO Secretion by DLBSA204
Production of NO in the normal RAW 264.7 cell was 14.59 pg/ml, whereas the cell culture that was co-cultured with L. fermentum DLBSA204 was measured at 36.35 pg/ml. The production of NO in cell co-stimulated with LPS (250 ng/ml) was increased to 48.58 pg/ml (fig. 2). Higher level of NO after stimulation of L. fermentum DLBSA204 compared to control indicated that macrophage has been activated.
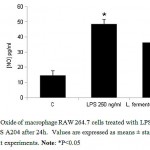 |
Figure 2: Nitric Oxide of macrophage RAW 264.7 cells treated with LPS 250 ng/ml and L. fermentum DLBS A204 after 24h. Values are expressed as means ± standard deviation of two independent experiments. Note: *P<0.05
|
Induction of S. pneumonia Phagocytosis in RAW 264.7 cells
In order to investigate the phagocytic activity of macrophage with L. fermentum DLBSA204 treatment, phagocytic activity assay was conducted. In this experiment, ciprofloxacin was used as an antibiotic that inhibit growth of external S. pneumonia. Fifteen minutes incubation of ciprofloxacin is the optimal time to ensure the survival of macrophage. In this study, DLBSA204 treated-cells have a higher phagocytic activity on S. pneumoniae with 2.5-fold higher activity compared to the normal cells (fig.3).
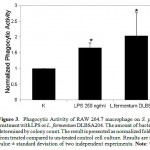 |
Figure 3: Phagocytic Activity of RAW 264.7 macrophage on S. pneumonia after treatment with LPS or L. fermentum DLBSA204. The amount of bacteria growth was determined by colony count. The result is presented as normalized fold value obtained from treated compared to un-treated control cell culture. Results are shown as mean value ± standard deviation of two independent experiments. Note: *P<0.05
|
Lysozyme measurement
Activated macrophage induces high level of lysozyme (23). This study measured the release of lysozyme from macrophage as an indicator of macrophage activation. Lysozyme level in the supernatant of L. fermentum DLBSA204 treated-macrophage cells was higher than the untreated cells (fig.4). Decreased turbidity was observed in the substrate buffer (as a measure of lysozyme) after treatment with supernatant of L. fermentum DLBSA204-treated cells. The lysozyme levels were measured in units/min.
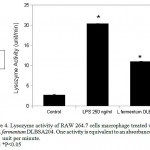 |
Figure 4: Lysozyme activity of RAW 264.7 cells macrophage treated with LPS and L.fermentum DLBSA204. One activity is equivalent to an absorbance decrease of 0.001 unit per minute. Note: *P<0.05
|
Effect of DLBSA204 to cytokine expression
Cytokine expression is an inducible marker of macrophage activation. To reveal whether macrophage activation by probiotic is dependent on TNF-α and IL-6 signal transduction, we traced the gene expression of macrophage after administration of LPS and DLBSA204. As shown in figure 5, after 24 h treatment, L. fermentum DLBSA204 induced higher level of TNF- α and IL-6 compared to control.
 |
Figure 5: Expression of several immunomodulatory signals in RAW 264.7 after treatment with LPS 250 ng/ml and L.fermentum DLBSA204 for 24h. (A) Shows PCR of TNF-α, IL-6 and GAPDH gene expression. Lane 1 shows gene expression from untreated cells, lane 2 from LPS 250 ng/ml treated-cells, lane 3 from L. fermentum DLBSA204 treated-cells. (B) Shows arbitrary unit (AU) of TNF-α gene expression. (C) Shows AU of IL-6 gene expression. Results are expressed as mean value ± standard deviation of two independent experiments. Note: *P<0.05
|
Effect of L. fermentum DLBSA204 on S. pneumoniae adherence
In order to examine the adhesion competition between L. fermentum DLBSA204 and S. pneumoniae, investigation was done using SEM photographs and total plate count method. In the control S. pneumonia and L.fermentum DLBSA204 samples, it was apparent that separately, the number of adherent S.pneumoniae to A549 cells was higher compared to the number of adherent L. fermentum DLBSA204 (fig. 6A, 6B, 6C & Table 2). In the co-inoculation samples, pre-incubation of A549 cells with L. fermentum DLBSA204 prior to S. pneumoniae reduced adhesion of S. pneumoniae to 3.19% (fig. 6D). Pre-incubation of A549 cells with S. pneumoniae prior to L. fermentum DLBSA204 also showed good result. The adhesion of S. pneumoniae was reduced to 9.82% (fig. 6E). This result proved that L. fermentum DLBSA204 is able to inhibit and remove pathogenic bacteria adhesion on the human lung cells.
 |
Figure 6: SEM observation on L.fermentum DLBSA204 and S.pneumoniae adherence to human lung cell. (A) A549 cells without bacteria adhesion. (B) A549 cells with adherent L.fermentum DLBSA204 for 24 hours. (C) A549 cells with adherent S.pneumoniae. (D) A549 cells incubated with L. fermentum DLBSA204 for 2 hours, followed by addition of S. pneumoniae for 24 hours. (E) A549 cells incubated with S. pneumoniae for 2 hours, then addition of L. fermentum DLBSA204 for 24 hours. Bar = 5 μm
|
Table 2: Adhesion of L.fermentum DLBSA204 and S.pneumoniae on human lung cells
| Treatment | % Adhesion to human lung cells | |
| L. fermentum DLBSA204 | S. penumoniae | |
| L. fermentum DLBSA204 only | 28.01 % | – |
| S. pneumonia only | – | 68.66 % |
| L. fermentum DLBSA204 then S. penumoniae | 30.81 % | 3.19 % |
| S. pneumonia then L. fermentum DLBSA204 | 67.79 % | 9.82 % |
Antioxidative Activity of L. fermentum DLBSA204
The antioxidative activity of L. fermentum DLBSA204 was determined by the percentage of inhibition to DPPH scavenging activity. Inhibition effect of L. fermentum DLBSA204 on DPPH radical was observed in a dose-dependent manner (Figure 7). The lowest dose of L. fermentum DLBSA204 (1/4 dose) in this experiment was 2×108 CFU/ml with 41.43% DPPH inhibition, while L. fermentum DLBSA204 109 CFU/ml inhibit 76.48% DPPH.
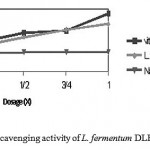 |
Figure 7: DPPH radical scavenging activity of L. fermentum DLBSA204
|
Discussion
The present study was performed to evaluate the potential of L.fermentum DLBSA204 as a mode of prevention and treatment of bacterial respiratory tract infection through immune response modulation. The study was conducted based on observations on three critical parameters; ability to activate macrophage, compete for adherence with S.pneumoniae and antioxidative activity (fig. 8).
Stimulation of innate immune system in initiating adaptive immune response is done by macrophage. Macrophages are tissue-based phagocytes which are derived from monocyte. Ability of macrophage to respond to environment stimuli by changing its form and physiological activity is referred to as “activation” response which is pivotal to the host immune function (24). Macrophage activation by L. fermentum DLBSA204 was investigated by measuring the proliferation, gene expression and biochemical activity of macrophage; which affect the innate and adaptive immune response. Different stimulus leads to different responses by the macrophage. In the present study, we found that L. fermentum DLBSA204 increased the number of macrophage. The increment was lower compared to those in the LPS- treatment, which was derived from E. coli (fig. 1). Stimulation by microorganism will result in Toll-like receptor (TLR) activation that induces expression of cytokine, such as TNF- α. Level of macrophage proliferation by L. fermentum DLBSA204 was in line with the level of upregulation of TNF-α gene expression (fig. 5A, fig. 5B). Upregulation of TNF-α gene is known to be a central key in immune response modulation because it is among the first cytokine produced by phagocytic cells, such as macrophage (25). This result suggests that macrophage proliferation was initiated by the upregulation of TNF-α gene expression (16).
Increment of TNF- α level will induce NF- КB pathway that produces NO as a marker for macrophage activation (24). NO production will increase in response to bacterial lipopolysaccharide. L. fermentum DLBSA204 treatment also increased NO production in macrophage although not as high as LPS treatment (fig. 2). Activated macrophage is indicated by its phagocytic activity (24). Phagocytosis is the primary means of removing foreign microorganism that is mediated by polymorphonuclear neutrophils and macrophages. In the lung, macrophage serves as effector cells which protect the alveolar surface from harmful microorganisms by phagocytosis (26). L. fermentum DLBSA204 was able to enhance the ability of macrophage to phagocyte S. pneumoniae (fig. 3).
Upregulation of TNF-α expression also increase lysozyme level (23). Lysozyme is a cationic enzyme that affects the β-1, 4 glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine in the peptidoglycan of bacterial cell wall. Lysozyme works by lysing the cell wall of Gram positive bacteria because it has thicker peptidoglycan layer compared to Gram negative bacteria (27). Pathogenic bacteria in the respiratory tract is not only limited to Gram positive bacteria, but also Gram negative bacteria. Lysis of other invasive Gram negative respiratory bacteria needs interaction of lysozyme with other defense protein, for example lactoferin (28). In this study, it was found that L. fermentum DLBSA204 enhanced secreted lysozyme level from macrophage (fig. 4). This result is similar to a study by Sun et al (2010) which showed that probiotic diet for 60 days increased level of lysozyme in rats (29).
Both pathogenic and probiotic bacteria adhere to epithelial cells of the host to survive and to further colonize the host. In this study, competition between both bacteria was evaluated to determine the ability of L.fermentum DLBSA204 in inhibiting respiratory tract infection by S.pneumoniae. The inhibition study was done by in vitro assay with human lung A549 cell as a model of human respiratory tract. The ability of L. fermentum DLBSA204 to inhibit pathogenic bacteria infection on human respiratory tract was examined in adhesion competition assay against S. pneumonia. S. pneumonia is a Gram-positive bacteria that induces nasopharyngeal and lung infection. It is a major pathogenic bacteria in human which has the ability to autolysis (30). This bacteria normally lives in mucosal surface of the upper respiratory tract. Infection will occur if the bacteria colonized sterile parts of the respiratory tract (11). The adhesion of L. fermentum DLBSA204 to A549 cell shows that L. fermentum DLBSA204 adhered to human lung cells with higher percentage compared to S. pneumoniae (Table 2). These data suggest that DLBSA204 was able to inhibit S.pneumonia from adhering to the human lung cells and replace adherent S. pneumoniae on the human lung cells. An in vivo experiment has been done and demonstrated adhesion competition between probiotic and pathogenic bacteria on respiratory tract ( 31). The result showed that Lactobacillus lactis NZ9000 was able to reduce colonization of S. pnuemoniae on the lung and prevent this pathogenic bacteria from entering the blood. The reduced number of pathogenic bacteria adherence is a result of competition for adhesion sites and nutritional sources, low pH effect from lactic acid, and secretion of antimicrobial substances (32). The ability to inhibit S. pneumonia from adhering to human epithelial cells was also demonstrated by Lactobacillus rhamnosus GG (LGG) (31). LGG was effective in inhibiting adherence in the pre- addition assay but less efficient in post-addition assay. The L. fermentum DLBSA204 works in a similar way as LGG. Pre-addition assay demonstrated prevention strategy, while post-addition assay demonstrated curative strategy. Therefore, it can be inferred that both LAB isolates work more effectively as preventive agent.
A double-blind, placebo-controlled study with 326 eligible children that consumed probiotics via oral route demonstrated that probiotic could reduce symptoms of upper respiratory tract infection (URTI (9). Reduced occurrence of cough symptoms was explained by enhanced immune response of the host. The probiotics improve local immunity (maintaining the integrity of gut wall) and enhance systemic immunity (enhancing non-specific immune system) (10). It was shown that administration of probiotic via oral route, rather than via nasal route also enhance immune response of the host.
One of several cytokines that is released by activated macrophage to induce other immune cells is IL-6. IL-6 gene expression was found to be higher after treatment with DLBSA204 (fig. 5A, 5C). IL-6 gene expression by macrophage initiated the activation of other immune cell responses, such as differentiation of B cells and T cells, and production of IgA (25). Elevated level of IL-6 gene expression observed in the present study further suggests that IL-6 was the key factor that caused IgA increment in animal after oral administration of probiotic.
Immune system is dependent to the level of antioxidant and oxidant in the body where antioxidant helps maintain cell membrane integrity and functionality, cellular proteins, and nucleic acids. Furthermore, it also helps control signal transduction and gene expression in the cells (33). Antioxidative activity of L. fermentum DLBSA204 was observed and it was found that L. fermentum DLBSA204 was able to increase the free radical scavenging activity in a dose-dependent manner (fig. 7). The antioxidative activity of DLBSA204 was measured by DPPH free radical scavenging method which shows the percentage of remaining DPPH when the kinetics reached a steady state (34). Several studies have shown that other probiotics, such as Lactobacillus paracasei (22), Lactobacillus plantarum (35) and Lactobacillus fermentum ME-3 (LfME-3) (36, 37) also have antioxidative activity ,.
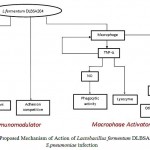 |
Figure 8: Proposed Mechanism of Action of Lactobacillus fermentum DLBSA204 on S.pneumoniae infection
|
Conclusion
Lactobacillus fermentum DLBSA204 has the potential to be used as an immunomodulator and macrophage activator. However, further study is needed to ensure the safety of this bacterium before it can be applied for human consumption.
Acknowledgment
The authors would like to thank Dr. Siswa Setyahadi and Hanna C. Rouli for their assistance in editing the manuscript. This research is supported by Dexa Medica.
References
- Pickering, K., Granoff, D.M., Erickson, J.R., Masor, M.L., Cordle, C.T., Schaller, J.P., Winship, T.R., Paule, C.L., Hilty, M.D. Modulation of the Immune System by Human Milk and Infant Formula Containing Nucleotides. Pediatrics, 1998; 101: 242-9
CrossRef - Garofalo, , Chheda, S., Mei, F., Palkowetz, K.H., Rudloff, H.E., Schmalstieg,F.C., Rassin, D.K., Goldman, A.S. Interleukin-10 in Human Milk. Pediatric Research, 1995; 37 (4): 444-9
CrossRef - Mehanna, N.S., Tawfik, N.F., Salem, M.M., Effat, B.A., El-Rab, D.A. Assessment of Potential Probiotic Bacteria Isolated from Breast Milk. Middle-East J. Scientific Research, 2013; 14 (3): 354-60
- Heikkila, M.P., Saris., P.E. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. Applied Microbiol, 2003; 95: 471- 8
CrossRef - Matsuzaki, T., Chin, J. Modulating immune responses with probiotic bacteria. Immunol and Cell Biol, 2000; 78: 67- 73
CrossRef - Ahrne, , Nobaek, S., Jeppsson, B., Adlerberth, I., Wold, A.E., Molin, G. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J. Applied Microbiol, 1998; 85: 88-94
CrossRef - Nasiraii, R., Tabatabaeie, F., Alaeddini, B., Noorbakhsh, R., Heravi, R.M., Afsharian, S. Investigation of lactobacilli from mother’s breast milk who were placed on probiotic diet. Afr. J. Microbiol. Res, 2011; 5 (13): 1581-5
- Jimenez, , Langa, S., Martin, V., Arroyo, R., Martin, R., Fernandez, L., Rodriguez, J.M. Complete Genome Sequence of Lactobacillus fermentum CECT 5716, a Probiotic Strain Isolated from Human Milk. J. Bacteriol, 2010; 192 (18): 4800
CrossRef - Leyer, J., Li, S., Mubasher, M.E., Reifer, C., Ouwehand A.C. Probiotic Effects on cold and influenza-like symptom incidence and duration in children. Pediatrics, 2009; 124: e172-e179
CrossRef - Hao, Q., Lu, Z., Dong, B.R., Huang, C.Q., Wu, T. Probiotics for preventing acute upper respiratory tract infections (Review). Cochrane Database of Systematic Reviews, 2011; Issue 9. Art. No.:CD006895. DOI:10.1002/14651858.CD006895.pub2
CrossRef - Cotton, M.F., Innes, S., Jaspan, H., Madide, A., Rabie, H. Management of upper respiratory tract infections in children. South African Family Practice, 2008: 50 (2):6-12
CrossRef - Kartasurya, M.I., Ahmed, F., Subagio, H.W., Rahfiludin, M.Z., Mark, G.C. Zinc combined with vitamin A reduces upper respiratory tract infection morbidity in a randomised trial in preschool children in Indonesia. British J Nutrition, 2012; 108: 2251–60
CrossRef - Harata, , He, F., Hiruta, N., Kawase, M., Kubota, A., Hiramatsu, M., Yausi, H. Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses. Letters in Applied Microbiol, 2010; 50: 597–602
CrossRef - He, F., Morita, H., Ouwehand, A.C. Bifidobacteria and lactobacilli exhibited different mitogenic activity on murine splenocytes. J Probiotics and Prebiotics, 2006; 1 (1): 77-82
- Maassena, C.B., Holten-Neelen, C., Balk, F., Bak-Glashouwera, M.J., Leer, R.J., Laman, J.D., Boersmac, W.J., Claassenc, E. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus Vaccine, 2000; 18: 2613-23
- Kim, W., Cho, S.B., Yun, C.H., Jeong, H.Y., Chung, W.T., Choi, C.W., Lee, H.J., Nam, I.S., Suh, G.H., Lee, S.S., Lee, B.S. Induction of cytokines and nitric oxide in murine macrophage stimulated with enzymatically digested Latobacillus strains. J Microbiol, 2007; 45: 373-8
- Chon, , Choi, B., Lee, E., Lee, S., Jeong, G. Immunomodulatory effects of specific bacterial components of Lactobacillus plantarum KFCC11389P on the murine macrophage cell line RAW 264.7. J Applied Microbiol, 2009; 107: 1588- 97
CrossRef - Laetitia, R., Khan, A., Paul, A., Coussa-Charley, M., Marinescu, D., Tomaro-Duchesneau, C., Shao, W., Kahouli, I., Prakash, S. Effect of probiotics Lactobacillus and Bifidobacterium on gut-derived lipopolysaccharides and inflammatory cytokines: an in vitro study using a human colonic microbiota model. J Microbiol. Biotechnol, 2013; 23(4): 518–26
CrossRef - Tjandrawinata, R.R., Byus, C.V., Regulation of the efflux of putrescine and cadaverine from rapidly growing cultured RAW 264 cells by extracellular putrescine. Biochem, 1995; 305 (1): 291-9
CrossRef - Tandrasasmita, O.M, Lee, J.S., Baek, S.H., Tjandrawinata, R.R. Induction of cellular apoptosis in human breast cancer by DLBS1425, a Phaleria marocarpa extract, via down-regulation of PI-kinase/AKT pathway. Cancer Biol Ther, 2010; 10 (8): 814-23
CrossRef - Tandrasasmita, O.M., Wulan, D.D., Nailufar, F., Sinambela, J., Tjandrawinata, R.R. Glucose-lowering effect of DLBS3233 is mediated through phosporylation of tyrosine and upregulation of PPARγ GLUT4 expression. Int J Gen Med, 2011; 4: 345-57
- Liu, , Pan, T. In Vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. J Food and Drug Analysis, 2010; 18 (2): 77-86
- C.E., McCarthy, S.P., Lorenzen, J., McGee, J.O. Differential effects of LPS, IFN-γ and TNFα on the secretion of lysozyme by individual human mononuclear phagocytes: relationship to cell maturity. Immunology, 1990; 69: 402-08
- Mosser, M, Zhang, X. Activation of Murine Macrophages. Curr Protoc Immunol, 2008: 1-9 DOI:10.1002/0471142735.im1402s83
CrossRef - Morita, , He, F., Fuse, T., Ouwehand, A.C., Hashimoto, H., Hosoda, M., Mizumachi, K., Kurisaki, J. Cytokine production by the murine marophage cell line J774.1 after exposure to Lactobacilli. Biosci Biotechnol Biochem, 2002; 66 (9): 1963- 6
CrossRef - Lee, , Serezani, C., Medeiros, A., Ballinger, M., Peters-Golden, M. 2009. Crosstalk between Prostaglandin E2 and Leukotriene B4 Regulates Phagocytosis in Alveolar Macrophages via Combinatorial Effects on Cyclic AMP1. J Immunol 182, pp. 530-7
CrossRef - Chassy, M., Giuffrida, A. Method for the lysis of gram-positive, Asporogenous bacteria with lysozyme. Applied and Environmental Microbiol, 1980; 39 (1): 153- 8
- Ellison, R.T., Giehl, T.J. Killing of Gram-negative bacteria by lactoferin and l J Clin Invest, 1991; 88, pp: 1080-91
- Sun, , Yang, H., Ma, R., Lin, W. Probiotic applications of two dominant gut Bacillus strain with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish & Shelffish Immunol, 2010; 29: 803- 9
CrossRef - Martner, , Skovbjerg, S., Paton, J.C., Wold, A.E. Streptococcus pneumonia autolysis prevents phagocytosis and production of phagocyte-activating cytokines. Infection and Immunity, 2009; 77 (9): 3826- 37
CrossRef - Wong, , Toh, Z.Q., Dunne, E.M., Mulholland, E.K., Tang, M.L., Robins-Browne, R.M., Licciardi. P.V., Satzke, C. Inhibition of Streptococcus pneumoniae adherence to human epithelial cells in vitro by the probiotic Lactobacillus rhamnosus GG. BMC Research Notes, 2013; 6:135-41
CrossRef - Lehto, M., Salminen, S.J. Inhibition of Salmonella typhimurium adhesion to Caco-2 cell cultures by Lactobacillus strain GG spent culture supernate: only a pH effect? FEMS Immunol and Med Microbiol, 1997; 18: 125- 32
CrossRef - Knight, J.A. Review: Free radicals, antioxidants and immune system. Ann Clin Lab Sci, 2000; 30 (2): 145 – 58
- Brand-Williams, , Cuvelier, M.E., Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm- Wiss. U- Technol, 1995; 28: 25- 30
CrossRef - Nedelcheva, , Denkova, Z., Denev, P., Slavchev, A., Krastanov, A. Probiotic strain Lactobacillus plantarum NBIMCC 2415 with antioxidant activity as a starter culture in the production of dried fermented meat products. Biotechnol & Biotechnological Equipment, 2010; 24 (1):1624-30
CrossRef - Mikelsaar, , Zimer, M. Lactobacillus fermentum ME-3 – an antimicrobial and antioxidative probiotic. Microbial ecology in health and disease, 2009; 21: 1- 27
CrossRef - Brambilla, , Mancuso, C., Scuderi, M.R., Bosco, P., Cantarella, G., Lempereur, L., Benedetto, G., Pezzino, S., Bernardini, R. The role of antioxidant supplement in immune system, neoplastic, and neurodegenerative disorders: a point of view for an assessment of the risk/benefit profile. Nutrition Journal, 2008; 7 (29) DOI:10.1186/1475-2891-7-29
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





