How to Cite | Publication History | PlumX Article Matrix
Nasrin Amouei1, Hedayat Sahraei2, Hengameh Alibeik1, Gholam Hossein Meftahi2, Zahra Bahari3 and Alireza Mohammadi2,*
1Department of Biology, School of Biological Sciences, Islamic Azad University, North Tehran Branch, Tehran, Iran.
2Neuroscience Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
3Department of Physiology and Biophysics, School of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran.
Corresponding Author E-mail: ar.mohammadi@bmsu.ac.ir
DOI : http://dx.doi.org/10.13005/bbra/2277
ABSTRACT: The hippocampus is one of the main parts of Limbic system and plays a crucial role in the response of brain to stress. In this study, the effect of nicotine injection on the CA1 region of the hippocampus of mice in responding to inescapable stress was studied. Fifty four adult NMRI male mice were divided randomly into nine groups, comprising a negative control group (without any intervention), two groups as positive controls (receiving 1 ml/mouse and 10 μg/mouse saline, respectively), three groups received Intrahippocampal (CA1) nicotine (getting 1, 5 and 10 μg/mouse nicotine) and three groups received intraperitoneal nicotine (receiving 0.25, 0.5 and 1 mg/kg nicotine). The water and food consumption, body weight, anorexia time, changes in weight of brain and adrenal glands were evaluated. Data were presented as mean standard error of mean (SEM) and analyzed using one-way analysis of variance (ANOVA). Body and brain weight, amount of food and water consumption were decreased following stress, but the adrenal gland weight and anorexia time were increased. The results showed that intraperitoneal administration of nicotine exacerbated the effects of stress in a dose of 1 mg/kg and reduced the effects of stress in doses of 0.5 and 0.25 mg/kg. Intrahippocampal injection of nicotine had no effect on the response to stress and only affects the brain's weight. We conclude that inescapable stress causes extensive changes in animal performance, which is related to nicotinic acetylcholine receptors present on hippocampal pyramidal neurons. On the other hand, nicotinic acetylcholine receptors may modulate the activity of glucocorticoid receptors in the hippocampus neurons and play an important role in moderating stress responses in a dose-dependent manner.
KEYWORDS: Stress; Nicotine; Hippocampus; Anorexia; Adrenal glands
Download this article as:| Copy the following to cite this article: Amouei N, Sahraei H, Alibeik H, Meftahi G. H, Bahari Z, Mohammadi A. Intrahippocampal and Peripheral Effects of Nicotine Injection on the Metabolic and Behavioral Response to Inescapable Stress. Biosci Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Amouei N, Sahraei H, Alibeik H, Meftahi G. H, Bahari Z, Mohammadi A. Intrahippocampal and Peripheral Effects of Nicotine Injection on the Metabolic and Behavioral Response to Inescapable Stress. Biosci Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15671 |
Introduction
Stress is a mental, emotional or physical influence that can initiate the “fight or flight” reaction, a complex response of neurobiological and neurochemical systems. Stress is one of the main reasons of accidents, chronic diseases and social problems such as addiction. Numerous studies have regarded the neurobiology of stress and the ways to reduce its side effects. It is well known that, stress hormones such as glucocorticoids and adrenaline are released in the blood following the stress and results in increasing blood pressure, heart rate and blood sugar. Long-term release of stress hormones in blood increases the risk of diabetes, metabolic and neurological disorders such as anxiety, depression and schizophenia [1]. Other studies showed that a significant percentage of stressed people suffer from chronic stress disorders including 3 million diabetic patients, 2 million addicts, 5-8 million smokers and also thousands of patients with heart and digestive diseases [2]. Anxiety, depression, decreased decision making skills and perilous behaviors are the results of chronic stress and they may cause social problems like addiction. In fact, stress is any mental or environmental factor that makes problems for living and the element that disrupts body homeostasis is called stressor [3]. In response to stress, homeostatic mechanisms in different systems of body start in order to support the individual’s life. One of the more common mechanisms is the activation of Hypothalamic-Pituitary-Adrenal (HPA) axis and increasing the level of Adrenocorticotropic hormone (ACTH) [1].
Main neurotransmitters contributed in stress and its responses to drugs include norepinephrine, 5-hydroxytiriptamin (5-HT or serotonin) and gamma-aminobutyric acid (GABA). Most of the neurobiological knowledge about stress is obtained from experiments of behavioral paradigms and effect of psychoactive drugs on animals [4]. Norepinephrine is a neurotransmitter that distributes in the central nervous system with the function in arousal, attention, stress, anxiety and their related disorders. Evaluation the effect of stress on adrenergic system in rats indicates that stress lead to increase in synthesis and release of norepinephrine in the brain [5]. Other studies indicated that stress decreases the intracellular synthesis of cAMP due to reduction of β-adrenergic receptors in cell membrane [6, 7]. It has been exhibited that stress reduced the sensitivity of β-adrenergic receptors to noradrenalin [6]. Mobley et al. (1984) reported that the sensitivity of β-adrenergic receptors to noradrenalin and the level of intracellular cAMP in rat brain increased following adrenalectomy [2].
Various types of acute stress lead to increasing of serotonin circulating in the prefrontal cortex, nucleus accumbens, amygdala and lateral hypothalamus. The cell bodies of most serotonergic neurons in Raphe nuclei are located in the dorsal brainstem and their axons project to the cortex, central core of amygdala, hippocampus and hypothalamus [8]. Evaluation of the effect of stress on central and peripheral serotonergic and dopaminergic system in rat showed that the level of serotonin in all over the brain and dopamine in specific parts of brain and plasma are increased [9]. Dopamine is the most studied neurotransmitter that have shown to play an essential role in the pathophysiology of schizophrenia [10]. The role of GABA in stress confirms the indisputable effect of benzodiazepines on treatment of anxiety disorders, which increase the effect of GABA on its receptors [2].
Nicotine is a drug that targets brain acetylcholine receptors. As nicotine binds to these receptors, the intracellular cation is increased and the cell becomes depolarized. Nicotine may cause increased dopamine distribution via regular activity on ionotropic receptors of GABA, dopamine and entrances of glutamatergic neurons [2]. Nicotine is quickly absorbed across respiratory tract, oral mucosa and skin and reaches the brain through a vein distribution within 8 seconds [11]. Nicotine is relatively a strong base so it’s absorption from the stomach depends on the rise in the stomach pH level but it absorbed easily through the intestine. Nearly 80-90 percent of nicotine is reformed in the liver and a bit lower in the kidneys and lungs [12]. Also, Nicotine is the most pharmacologically active compound in tobacco and psychoactive substances [13-16] and its pharmacological effects are due to inducing the release of various neurotransmitters [17]. It has been shown that smoking reduces anxiety due to nicotine withdrawal [18]. Chronic stimulation of the sympathetic nervous system by nicotine increases metabolic rate and the metabolism of brown fat and results in weight loss [13, 18]. Nicotine is defined as an effective drug to treat various diseases [14]. It has been reported that acute systemic administration of nicotine improves the memory in animals with cognitive problems [19]. It also enhances dynamic activity and food related conditioned-reflex [11]. Most smokers report that daily smoking helps them to focus and relax so they can work more effectively. In human, positive reinforcing effects of nicotine include mild euphoria, increased energy, exacerbated mobility, decreased blood pressure, anxiety and appetite [11]. In rat, it has been shown that intraperitoneal administration of nicotine significantly influences place preference [20].
Considering the costly care and treatment of stressed people, new investigations would be done to reduce the incidence of stress and its side effects on society. Therefore, drugs that can reduce these effects may act as a stress-reducing agents and protect individuals against the prolonged effects of stress. In this research, was studying the nicotinic cholinergic system interference, especially in the hippocampus on remission or the exacerbation of inescapable effects of stress in mice.
Materials and Methods
Animals
In this study, adult NMRI male mice (25-35 gr, the Pasteur Institute, Tehran, Iran) were used during the study. Animals were studied in a period of 1-2 weeks. They were exposed to stress at different times of day. During the stress period (7 days), two days were randomly stress-free. After the recovery period of 5 to 7 days, experiments were started.
In the practical phase of the study, fifty four mice were divided randomly into nine groups, including a negative control group (Control–, without any intervention), two groups as positive controls (Control IP+ and Control Hipp+, receiving 1 ml/mouse and 10 μg/mouse saline, respectively), three groups received intraperitoneal nicotine (IP Nic, receiving 0.25, 0.5 and 1 mg/kg nicotine) and three groups received Intrahippocampal (CA1) nicotine (Hipp Nic, getting 1, 5 and 10 μg/mouse nicotine).
Drugs
The nicotine was dissolved in sterile saline before use and injected into the peritoneum (0.25, 0.5 and 1 mg/kg) or hippocampus (1, 5 and 10 μg/mouse).
Experimental methods
After the adaptation period (7 days), the stress period (7 days) was started as follows: On the first day, the animals were weighed and nicotine solution was injected intraperitoneally based on their body weight. Thirty minutes were given the drug to reach the desired effect, then the animals were placed in the stress box. Stress box is a Plexiglas device which contains 9 rooms. Each room is connected with others via small holes that allow animals to communicate with each other by smelling and hearing. The transparent walls of the rooms allow visual communication of animals. The bottom of the device consists of stainless steel bars that connect to an electric shock generator or electroshock device (ESD). ESD generates electrical current which is transmitted through a cable to the stress box. The voltage, frequency and time can be adjusted on this device.
Animals were placed in stress box and allowed to adapt with the box for 20 minutes. They were exposed to an electric current for 60 seconds at a voltage of 60V and frequency of 10 hertz (HZ). Then the mice were given a 10 minutes rest. After that, animals were placed in the storage container and given water and food. Since the mice were kept in a box until the first mice reach the food was considered as anorexia. After the experiments, they were taken to the animal house. This procedure was repeated for 7 days at random times of the day. The animals were not receiving any stress for randomly selected 2 days in this interval of 7 days, this caused inescapable of stress to the animals. The weight of animals and the amounts of food and water consumption were calculated at a particular time each day. This process was repeated for other control and hippocampal groups.
Surgical Procedures
To inject nicotine or saline into the hippocampus (CA1), the animals were first anesthetized by IP injection of ketamine (50–75 mg/kg) and xylazine (5–7 mg/kg). A stainless steel guide cannula (Gauge 21) was placed within the CA1 (the stereotaxic coordinates were AP = −4.5 mm, ML = +/-2.5 mm, DV = 2.5 mm from bregma, Fig 1). Injection into the CA1 was performed by a stainless steel injection cannula (Gauge 30) which was attached to a 5 µL Hamilton syringe by a plastic cannula. After finishing the experiments, animals were deeply anesthetized with ketamine (100 mg/kg) and their brains and adrenal glands were excised, fixed using 4% formalin and their weights were measured.
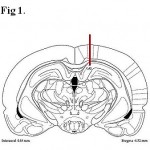 |
Figure 1: Location of the guide cannula in the CA1.
|
Data analysis
We evaluated the changes in body weight and water and food intake following stress induction. The size of the adrenal glands and brain and anorexia time were also calculated. Data were presented as meanstandard error of mean (SEM) and analyzed using one-way analysis of variance (ANOVA) followed by an LSD test to analyze changes in brain’s and adrenal gland’s weight and Repeated Measures ANOVA to analyze changes in body weight, food and water intake. P < 0.05 was considered as an indication of a significant difference.
Results
Effects of intraperitoneal administration of nicotine
Effects of intraperitoneal administration of nicotine on body weight and food intake
The body weight of Control– group increased by 0.5 mg/kg injection of nicotine whereas the Control IP+ group with administration of 0.25 and 1 mg/kg nicotine, lost body weight. Generally, the increasing or decreasing alterations in body weight were significantly different between groups (P <0.0001) meaning that weight changes depend on the dose of nicotine and that the animals are stressed or not. (Fig 2A)
Control–, Control IP+ and stressed groups showed an increase in food intake. The greatest increase was observed in the group received 1mg/kg nicotine (IP Nic 1) and the lowest one was seen in IP Nic 0.25 (Fig 2B).
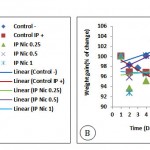 |
Figure 2: The amount of food intake (A) and average body weight (B) in mice under stress with and without nicotine injection (P0.0001, for food intake and weight changes).
|
Effects of intraperitoneal administration of nicotine on brain weight and water intake
The average brain weight in the Control IP+ and groups received nicotine decreased significantly compared with the Control– group (P <0.0001) (Fig 3A). The results showed that increased water intake in groups received nicotine were lower than Control IP+ group (Fig 3B).
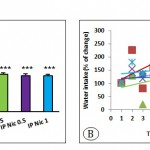 |
Figure 3: The average brain weight (A) and amount of water intake (B). (P0.0001, for brain weight and water intake).
|
Effects of intraperitoneal administration of nicotine on adrenal glands weight and anorexia
The results showed that the average weight of the adrenal glands in the stressed group received 1 mg/kg nicotine was significantly different from the Control– group (P<0.05, Fig 4A). The effect of different doses of intraperitoneally administered nicotine on the appetite (or anorexia) in stressed and Control– is shown in Fig 4B. The appetite depends on the dose of nicotine and that the mice was stressed or not.
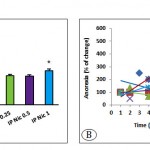 |
Figure 4: The average adrenal gland weight (A) and anorexia (B) in the positive and negative control groups and stressed groups received different doses of nicotine (P<0.05 for adrenal weight (*) and P0.0001 for anorexia).
|
Effects of bilateral intrahippocampal injection of nicotine
Effects of bilateral injection of nicotine in hippocampal area ca1 on body weight and food intake
The Control– group and stressed groups received nicotine in the area CA1; showed weight gain, which was highest in mice received nicotine with a dose of 5 μg/mouse (Hipp Nic 5). The body weight was decreased in the Control Hipp+ group (Fig 5A). The Control Hipp+ group showed the highest rate of food intake followed by Hipp Nic 1, Hipp Nic 5, Hipp Nic 10 stressing groups (Fig 5B).
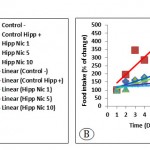 |
Figure 5: The average body weight (A) and amount of food intake (B) in mice under stress with and without intra-hippocampal injection of nicotine. (P0.0001, for weight changes and food intake).
|
Effects of bilateral injection of nicotine in hippocampal area CA1 on brain weight and water intake
According to the results, the level of water intake depends on doses of nicotine and whether the animals are under stress or not. The water intake in the Control– group and stressed group received 10 μg/mouse nicotine in hippocampal area CA1 remained almost constant. The Hipp Nic 1 showed a decrease in water intake and Control Hipp+ and the Hipp Nic 5 showed an increase in water intake (Fig 6A). Average brain weight following bilateral injection of nicotine in the CA1 area did not any significant difference between groups. (Fig 6B).
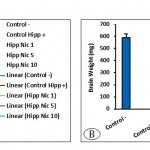 |
Figure 6: The amount of water intake (A) and average brain weight (B). (P0.0001, for water intake and brain weight).
|
Effects of bilateral injection of nicotine in hippocampal area CA1 on adrenal gland weight and anorexia
The Hipp Nic 1, Hipp Nic 5, Hipp Nic 10 groups, showed a significant increase in average weight of adrenal glands compared with the Control– group (P<0.001, Fig 7A). The Control– group showed more reduction in anorexia time (increased appetite) than the Control Hipp+ and Hipp Nic 10. Increased anorexia following injection of nicotine with those of the 1μg/mouse (Hipp Nic 1) was higher than doses of 5 μg/mouse (Hipp Nic 5) in stressed groups. The results showed that the desire to food intake depends on the dose of nicotine, and whether the animals are under stress or not (Fig 7B).
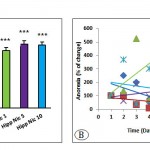 |
Figure 7: The average adrenal gland weight (A) and anorexia (B) in positive and negative control groups and stressed groups received intra-hippocampal different doses of nicotine. (P<0.05 (*) and P<0.001 (***) for adrenal weight and P0.0001 for anorexia).
|
Discussion and Conclusion
In this study, we evaluated the stimulation of nicotinic acetylcholine receptors in the hippocampus and its effect on inescapable stress responses in mice. In line with previous studies, the results of our study have showed that inescapable stress causes extensive changes in animal performance which is related to reduced food intake, increased water intake, brain weight loss and sharp increase of adrenal gland weight [21]. Increase in anorexia and decrease in body weight were also observed in stressed animals demonstrating the effects of stress on animal’s neurobiological mechanisms which is consistent with the results of other researchers in this field [22-27].
It should be noted that according to previous studies, stress stimulates HPA axis and increases secretion of glucocorticoids and epinephrine from the cortical and central parts of adrenal gland, respectively [21, 23]. Researches have shown the reduced level of dopamine in hippocampus following inescapable stress, which improves the animal’s ability to get used to stress [22, 27, 28]. Different parts of reward system are involved in the modulation of stress responses and the hippocampus as one of the main parts of this reward system plays an important role in controlling effects of stress [28].
Hippocampal neurons have a high density of glucocorticoid receptors on their surfaces which are activated in response to stress hormones secreted by adrenal glands; these neurons respond to stress as changes in the cell’s size and morphology and consequently the morphology and size of the hippocampus [23]. On the other hand, stimulation of nicotinic acetylcholine receptors present in hippocampus leads to improved efficiency of main neurons in this region [21]. In the present study, the effects of nicotinic receptors in hippocampus on responses to inescapable stress were examined in animals. It is well known that stress induces metabolic and behavioral responses through effects on the other parts of brain such as amygdala, accumbens nucleus and hypothalamus [23], thereby the behavioral responses we have observed in control groups does not have much differences with stress groups that is similar to previous findings. Our study indicated that stress decreases brain weight in the control group (saline administration in the hippocampus, Control Hipp+), while nicotine modulates this effect in a dose-dependent manner. On the other words, stimulation of nicotinic receptors may modulate the activity of glucocorticoid receptors in the hippocampus neurons. Nicotinic receptors on neurons of the mammalian brain belong to a family of receptors expressed by 11 genes (α2-α7, α9, α10 and β2-β4). The α-subunits of receptors are the major binding sites on nicotinic receptors [2]. Typically five numbers of subunits come together to form a central cationic channel. Stimulation of the receptor allows the cationic channel to transport cations (sodium, potassium or calcium). It appears that administration of nicotine may inhibit the effects of stress on the nervous system (particularly hippocampus) via stimulation of α2-β4 or α7 nicotinic receptors. Recent investigations have shown that the interaction between nicotine and stress is directly linked to α2 nicotinic receptors on pyramidal and GABAergic neurons in the hippocampus [23].
It is well established that all types of nicotinic acetylcholine receptors mRNAs are expressed in the hippocampus and α7 receptor is the most abundant one. While most of α7 nicotinic receptors in the hippocampus are located on interneurons in striatum radiatum, other types of nicotinic receptors like α2-β4 receptors are located on interneurons in other parts like striatum oriens [2]. The main interference has been presented between nicotine and α7 receptors in the hippocampus. In this regard, it has been shown that most responses to stress in the brain cholinergic system mediated by α7 receptors which may even affect the Chromaffin cells in the central part of the adrenal gland [29]. In addition to different distribution of nicotinic acetylcholine receptors in several neurons, their distribution is not same in all parts of a neuron (cell body, dendrites and synaptic terminals) [21]. Stimulation of nicotinic α7 receptors located on the terminal branches of hippocampal mossy fibers in mice, increases release of glutamate and activation of related mechanisms such as calcium-calmodulin kinase II and flow of calcium ions into the cell followed by development of cumulative potentials and synaptic plasticity [29].
The α7 receptors located on cell body and dendrites of hippocampal interneurons, modulate their activity by inhibiting the release of GABA [21]. Nicotinic acetylcholine receptors present on hippocampal pyramidal neurons may play an important role in modulating stress responses as shown in this study. Nicotinic α7 receptors on astrocytes in the hippocampus allow the entrance of calcium ions into the neurons, which in turn increases the activity of astrocytes and stimulation of local synapses, consequently is likely nicotine in this way could also cause deformation in the synapses [21, 22, 26, 29].
Several studies have described various responses of nicotinic acetylcholine receptors and their subunits to nicotine. While α7 nicotinic receptors rapidly lose their sensitivity to nicotine, α4-β2 receptors lose their senses slowly. As α4-β2 receptors are tendentious to nicotine more than α7 receptors, these receptors are the first ones to become insensitive at low doses of nicotine [30]. There is a relationship between stimulation of hippocampal nicotinic receptors and pyramidal neuron’s activity as nicotinic receptors may enhance the activity of these neurons and lead to increased memory power in living beings. Previous explores showed that nicotinic receptors may play a prominent role in some diseases which are directly related to stress. Diseases such as Autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) which is exacerbated by stress, is associated with mutation in α4 and β2 subunits. However, injecting proteins produced by these mutated genes to the Xenopus oocyte, the related receptors become readily insensitive and lose their permeability to calcium. Mice expressing mutant receptors, show behaviors similar to ADNFLE after exposure to nicotine [21]. Furthermore, stimulation of nicotinic acetylcholine receptors improves memory and attention in humans, which is observed in Alzheimer patients who smoke, indicating the interference of hippocampus and nicotine in a wide range of stress-related diseases. α7, α9 and α10 subunits of nicotinic acetylcholine receptors are present in cholinergic nerve terminals in the central part of the adrenal glands [27].
A recent study evaluating the effects of stress in the presence of corticotropin-releasing hormone (CRH) receptor’s inhibitors indicated that administration of CRH in presence or absence of CRH receptor’s antagonist increases or reduces CRH secretion [2]. Previous research has shown that nicotine may affect nicotinic receptors in hippocampus and consequently delay β-amyloid protein production in Alzheimer’s disease [21]. Moreover, it was demonstrated that glucocorticoids affect their receptors in the hippocampus especially mineralocorticoid receptors and stimulate the production of β-amyloid related enzymes [23]. Our research demonstrated the interaction between these two important pathways to induce or inhibit the effects of stress. In conclusion, this study has explained the strong impressionability of hippocampus from nicotine and nicotine’s effect on the events occurred following stress in hippocampus.
References
- F Ailing, et al. Role of extracellular signal-regulated kinase signal transduction pathway in anxiety. J Psychiat Res 2008. 43(1): p. 55-63.
CrossRef - A Aleisa, K Alzoubi, and K Alkadhi. Chronic but not acute nicotine treatment reverses stress-induced impairment of LTP in anesthetized rats. Brain Res.2006. 1097(1): p. 78-84.
CrossRef - L Alvarez‐Jaimes, D G Stouffer, and L H Parsons. Chronic ethanol treatment potentiates ethanol‐induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J. Neurochem.2009. 111(1): p. 37-48.
CrossRef - R H Baisden, M L Woodruff, and D B Hoover. Cholinergic and non-cholinergic septo-hippocampal projections: a double-label horseradish peroxidase-acetylcholinesterase study in the rabbit. Brain Res.1984. 290(1): p. 146-151.
CrossRef - D Balfour. The effects of nicotine on brain neurotransmitter systems. Pharmacol Therapeut.1982. 16(2): p. 269-282.
CrossRef - D J Balfour, et al. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Beh.1998. 59(4): p. 1021-1030.
CrossRef - R T Bartus, et al. The cholinergic hypothesis of geriatric memory dysfunction. Science.1982. 217(4558): p. 408-414.
CrossRef - M Sanchez, C O Ladd, and P M Plotsky. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol.2001. 13(3): p. 419-449.
CrossRef - Z I Bashir, et al. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature.1991.
CrossRef - M Noori-Daloii, et al. Knocking Down the DRD2 by shRNA Expressing Plasmids in the Nucleus Accumbens Prevented the Disrupting Effect of Apomorphine on Prepulse Inhibition in Rat. J. Sci. I. R. Iran.2015. 26(3): p. 205-212.
- N L Benowitz, et al. Stable isotope studies of nicotine kinetics and bioavailability. Clin. Pharmacol. Ther.1991. 49(3): p. 270-277.
CrossRef - J A Oates, A J Wood, and N L Benowitz. Pharmacologic aspects of cigarette smoking and nicotine addiction. New Eng J Med.1988. 319(20): p. 1318-1330.
CrossRef - H Betz. Homology and analogy in transmembrane channel design: lessons from synaptic membrane proteins. Biochemistry.1990. 29(15): p. 3591-3599.
CrossRef - A Bidzseranova, J Varga, and G Telegdy. The effects of receptor blockers on brain natriuretic peptide-32-induced action on passive avoidance behavior in rats. Neuropeptides.1992. 22(2): p. 107-10.
CrossRef - E L BLISS and J AILION. Response of neurogenic amines to aggregation and strangers. J. Pharmacol. Exp. Ther.1969. 168(2): p. 258-263.
- A Blokland, et al. Local inhibition of hippocampal nitric oxide synthase does not impair place learning in the Morris water escape task in rats. Eur. J. Neurosci.1999. 11(1): p. 223-232.
CrossRef - M T N Bombig, et al. Pravastatin protection from cold stress in myocardium of rats. Jpn. Heart J.2003. 44(2): p. 243-255.
CrossRef - C L Bon and J Garthwaite. On the role of nitric oxide in hippocampal long-term potentiation. J. Neurosci.2003. 23(5): p. 1941-1948.
CrossRef - J A Boscarino, et al. Association of FKBP5, COMT and CHRNA5 polymorphisms with PTSD among outpatients at risk for PTSD. Psychiat Res.2011. 188(1): p. 173-174.
CrossRef - S Hosseini, et al. Inactivation of the Nucl. Accumbens Core Exerts No Effect on Nicotine-Induced Conditioned Place Preference. Neurophysiology.2015. 47(4): p. 295-301.
CrossRef - T Brenner, et al. Evidence that central nicotinic-acetylcholine receptors are involved in the modulation of basal and stress-induced adrenocortical responses. Exp. Neurol.1986. 94(3): p. 735-743.
CrossRef - S Budavavi, et al., An encyclopedia of chemicals, drugs, and biologicals. 1996.
- A R Caggiula, et al. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology.2002. 163(2): p. 230-237.
CrossRef - B J Caldarone, S L King, and M R Picciotto. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neuroscience letters.2008. 439(2): p. 187-91.
CrossRef - J Cao, et al. Locomotor and stress responses to nicotine differ in adolescent and adult rats. Pharmacol Biochem Be.2010. 96(1): p. 82-90.
CrossRef - M Carpenter, Core text of neuroanatomy Williams and Wilkins. 1991, Baltimore, MD. 361-389.
- J-P Changeux, et al. Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Res. Rev.1998. 26(2): p. 198-216.
CrossRef - J-P Changeux, et al. New mutants to explore nicotinic receptor functions. Trends Pharmacol. Sci.1992. 13: p. 299-301.
CrossRef - Y Chen, D Graham, and T Stone. Release of endogenous adenosine and its metabolites by the activation of NMDA receptors in the rat hippocampus in vivo. Brit J Pharmacol.1992. 106(3): p. 632-638.
CrossRef - A Chiari, et al. Sex differences in cholinergic analgesia I: a supplemental nicotinic mechanism in normal females. Anesthesiology.1999. 91(5): p. 1447-54.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





