How to Cite | Publication History | PlumX Article Matrix
Potential of Echium ameonum Fisch and Mey in Removing Heavy Metals from Pharmaceutical Effluent
Afra Jafari1, Gholamreza Amin2 and Parisa ziarati*3
1Pharmaceutical Sciences Research Center, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS), Tehran, Iran.
2Department of Pharmacognosy, Faculty of Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS), Tehran, Iran.
3Department of Medicinal Chemistry, Faculty of Pharmacy, Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS), Tehran, Iran.
Corresponding Author E-mail: ziarati.p@iaups.ac.ir
DOI : http://dx.doi.org/10.13005/bbra/2303
ABSTRACT: The environmental and physical consequences of improper pharmaceutical waste management are serious. Hazardous chemical materials and Pharmaceutical waste from educational laboratory systems usually is thrown into the trash or dumped into a sink and thereby placed in the sewer waste stream. If these chemical compounds are not biodegraded or eliminated during sewage treatment, they eventually reach drinking water. In fact, most sewage and water treatment facilities do not take pharmaceutical contaminants into consideration, so these wastes are left untreated to enter our surface, ground, and drinking water. The main waste streams that educational sites of pharmaceutical Sciences branch, Islamic Azad University, Tehran were Cobalt and Cadmium compounds. The majority of the effluent from laboratories consists primarily of a mixture of water and acid and as its toxicity to the staff; they were confined to fume hoods. Information regarding the extent and concentration of all the chemicals expected to be used in the laboratory were mainly obtained from the end user. These chemicals have founded their way into the drain pipe. Due to vast medicinal benefits of Echium amoenum Fisch & C.A. Mey and availability of its dried flowers in Tehran market, we chose it for cleaning –up the soil. E. amoenum dried flowers added as an adsorbent in soil samples under different experimental conditions in this study and have been studied after every 3 days in 30 days. Aerial parts of growing basil in every three days in companion of E. amoenum fruits in the soils were separated in 3 days and digested by wet method according the standard protocol for measuring Cadmium ,Chrome (III) and (VI) , Nickel and Lead. Mean values were calculated, and the data were analyzed using Analysis of Variance, completely randomized block design (ANOVA). Results revealed that E. amoenum flowers have more potential to absorb Cobalt than Cadmium first days of study (p<0.003). The present study focused on adsorption capacity of Cr (VI), Cr (III), Co and Cd , Ni and Pb by E. amoenum was investigated in a batch system by considering the effects of various parameters like contact time, initial concentrations, pH , temperature, absorbent dose. The results of this study revealed that E. amoenum fruit can accumulate high level of Cobalt, Chrome (VI) and (III) in a short time and their uptake rate by vegetable and edible plant is significantly affected by their concentrations in the contaminated soil (p<0.05). A contact time of 15 days by E. amoenum was found to be optimum and 90.6% Cr (VI), 82.3% Cr(III),78.9% Co, 70.3% Pb and 48.6% Ni was taken by adsorbent from contaminated soil while a few amounts of these heavy metals being uptake by edible vegetable – basil. To quantify the occurrence and the distribution of heavy metals, to evaluate their effects, and to prevent them from passing through wastewater collection and treatment systems into soil and ground water bodies represents an urgent task for applied environmental sciences in the coming years. Public acceptance of green technologies is generally higher than that of industrial processes. The responsible organizations should stimulate research to upgrade existing waste water treatment by implementing phytoremediation modules and demonstrating their reliability to the public.
KEYWORDS: Pharmaceutical effluent; Adsorbent; Clean-up Soil; Echium ameonum Fisch and Mey
Download this article as:| Copy the following to cite this article: Jafari A, Amin G, ziarati P. Potential of Echium ameonum Fisch and Mey in Removing Heavy Metals from Pharmaceutical Effluent. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Jafari A, Amin G, ziarati P. Potential of Echium ameonum Fisch and Mey in Removing Heavy Metals from Pharmaceutical Effluent. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15924 |
Introduction
Pharmaceutical waste is one of the major complex and toxic industrial wastes [1, 2]. Pharmaceutical wastewater could be physicochemical treated by some methods such as bio-sorption, adsorption, screening, equalization, neutralization/pH adjustment, coagulation/flocculation, sedimentation, ozone and hydrogen peroxide treatment. Pharmaceuticals’ wastes fall into the emerging toxicants and pollutants category and need special attention. These pollutants are currently undergoing a regularization process although the directives and legal frameworks are not set-up yet [3]. Pharmaceuticals find their way into the environment via human and animal excreta from disposal into the sewage system [4]. Their presence in water can also be attributed to pharmaceutical industry waste, hospital waste and therapeutic drugs [5]. They are not only released into the environment after use. Some are disposed during manufacture or as unused or expired drugs [6].
Wastewater generated from the pharmaceutical laboratories varies dramatically in ranging from acidic to alkaline and pH. For example, the pH of an alkaline waste stream from a synthetic organic pharmaceutical plant ranges from 9 to 10, whereas a pH of 0.8 has been reported for acidic waste streams [7, 8]. Nevertheless, almost all types of waste streams produced from the pharmaceutical industry and research laboratories are either alkaline or acidic, and require neutralization before biological treatment. Thus, neutralization/pH adjustment of the waste prior to the biological system is a very important treatment unit for the biological treatment of pharmaceutical wastewater. The pH of the wastewater in this unit is adjusted by adding alkali or acid depending upon the requirement of the raw wastewater.
Significant parameters should be considered in designing a treatment for pharmaceutical wastewater. Biochemical oxygen demand determination of the waste must be considered with dilution, indicating the presence of toxic or inhibitory substances in some pharmaceutical effluents[9,10]. Pretreatment and recovery of various useful byproducts such as solvents, acids, sodium sulfate, fermentation solids, and fermentation beers comprise a very important waste control strategy for pharmaceutical plants. Such an approach not only makes expensive biological treatment unnecessary, but also gives economic returns in recovery of valuable byproducts [11-16]. Pharmaceutical wastewater contains various kinds of recalcitrant organics such as toluene, phenols, nitrophenols, nitroaniline, trichloromethyl propanol (TCMP), and other pollutants that exhibit resistance against biodegradation. Since these pollutants cannot be easily removed by biological treatment, biologically treated effluent exhibits a considerable oxygen demand, that is, BOD and COD, in the effluent. It has also been reported that activated carbon adsorption may not always be successful in removing such recalcitrant organics [17 -18]. Economic constraints may also prohibit the treatment of pharmaceutical wastewater by activated carbon adsorption [19]. In such cases, ozone/hydrogen peroxide treatment may appear to be a proven technology for treating such pollutants from pharmaceutical wastewater.
The possibility of treatment of pharmaceutical waste water combined with other industrial waste has been explored and evaluated [20]. One study carried out nitrification of high-strength nitrogenous wastewater (a concentrated stream from a urea plant) in a continuously stirred tank reactor. Pharmaceutical wastewater was used as an organic carbon source to maintain a COD/TKN ratio of 1. Such treatment alternatives establish the advantages of a dual mechanism of treatment, that is, nitrification as well as oxidation of organic pollutants.
The environmental pollution with toxic metals has become a worldwide crisis, affecting agriculture and contributing to bioaccumulation and biomagnifications in the food chain. Recently, research groups have recognized that certain toxic metals may remain in the environment for a long period and can eventually bio-accumulate to higher levels that could affect human being. Plants show some ability to reduce the hazards of organic pollutants [19-24], the greatest progress in phytoremediation has been made with metals. In this study Echium amoenum Fisch & C.A. Mey is one of the most important medicinal plants in Iranian traditional medicine selected . The flowers of this plant have been used as demulcent, anti-inflammatory and analgesic, anxiolytic, and sedative in folk medicine of Iran [25-26]. It is one of the famous plants in Iranian traditional medicine [27]. E. amoenum is well known for different kind of effect such as demulcent, anti-inflammatory, analgesic, anxiolytic and sedative properties in folk medicine of Iran [27,28]. E. amoenum is distributed in a wide area from northern Iran including the altitudes of Roudsar (Eshkevarat), altitudes between Roudbar and Manjil (including northern parts of Harzavil), altitudes of Amarlou (Charmkesh, Kaboutarchak, Damash, Ispili), altitudes of Siahbishe (road of Karaj to Chalous), altitudes of Heiran between Ardabil and Astara, Kandovan and Pole-Zangoule forest, Chaloos valley Mirmahalleh , between Gorgan and Navadeh and in the hillsides of Alborz ranges widely, as well it is cultivated in these areas in limited scale [ 29- 44]. Present study also was performed aiming to investigate effective strategies to remove toxic heavy metals by E. amoenum . On the whole the main aim of the current study is to: Assess the applicability of Echium amoenum Fisch & C.A. Mey in removing heavy metals from the contaminated soil .
Materials and Method
Pharmaceutical research laboratories effluent samples were collected between 1 April and 1 December 2015. Sample collection containers (1 L, amber glass) were washed in hot water, rinsed three times with distilled water, rinsed three times with acetone, and then baked in a heated oven at 250ºC for a minimum of four hours. A 24-hour composite sample (500 mL of effluent) was collected by WWTP operators from each WWTP, using their own equipment, and 100 2 mL of a solution containing 5.0 g/L of Na2EDTA and 25 mg/L of L. ascorbic acid (99% sigma-aldrich ) was added at the time of collection. The samples were shipped overnight on wet ice, and stored at 4°C until extraction. Because of the large number of sampling sites and chemical analytes, it was logistically too difficult and expensive to collect and analyze field blanks as well as duplicates from each location. Field blanks were collected from 20% of the sampling sites, with the field blanks being prepared from laboratory distilled water that was transferred into sampling containers and preserved at the time of collection. Duplicates were collected and analyzed for 10% of the sample sites.
Waste water Effluent
Effluents from 10 educational and research laboratories in pharmaceutical sciences branch, Azad university in Tehran, including, Food Science and Technology research (Effluent 1-4), Toxicology (Effluent 5-8), Analytical chemistry (Effluent 9,10) were used in this study. Effluent 1, 2,3 and Effluent 4 were from the same laboratory but were collected on separate occasions with a 3 week time interval. Although these effluents come from the same WWTP, they were treated as 4 different effluents due to the variability of their characteristics. This difference is attributed to the significant experiments which occurred following the first sampling event. After collection, the effluent was immediately transported to the research laboratory for analysis. Physico-chemical parameters such as pH, Electrical Conductivity, Total Solids, Total Dissolved Solids, Total hardness, Chloride, Sulphate, Dissolved oxygen, , Calcium, Sodium, Cadmium, Lead, Zinc, Copper, Chrome, Manganese ,Iron and Potassium were analyzed as per the standard methods [45].
The initial concentration of heavy metals/metalloid in the plants and effluents were analyzed before introduction into the studied soil samples. After 3days of treatment up to 30 days in every three days , final concentration of heavy metals/metalloid in effluent samples and plants were analyzed using Atomic Absorption Spectroscopy . The samples were analyzed by an Atomic Absorption Spectrophotometer Model AA-6200 (Shimadzu, Japan) using an air-acetylene flame for heavy metals and using at least five standard solutions for each metal. All necessary precautions were taken to avoid any possible contamination of the sample as per the AOAC guidelines [46-47].
Estimation of Heavy Metals in Effluents
The heavy metals/metalloid in the effluent samples such as Chrome, Zinc, Copper, lead, cadmium, Manganese and Iron were analyzed before and after treatment by AAS after digestion of plant materials by AOAC method31-32. The plant samples were washed in deionizer water dried (24 hrs at 80°C) immediately to stabilize the tissue and stop enzymatic reactions. After drying, samples were ground to pass a 1.0mm screen using the appropriate Wiley Mill. After grinding, the sample were thoroughly mixed and a 5- to 8-g aliquot withdrawn for analyses and storage33. Weighed 0.5 to 1.0 g of dried (80°C) plant material that has been ground (0.5 to 1.0 mm) and thoroughly homogenized and place in a tall-form beaker or digestion tube. Added 5.0 ml concentrated HNO3 (65 %) and cover beaker with watch glass or place a funnel in the mouth of digestion tube and allow to stand overnight or until frothing subsides. Place covered beaker on hot plate or digestion tube into block digester and heat at 125°C for 1 hour. Removed the digestion tube and allowed cooling. Added 1 to 2 ml 30% H2 O2 and digest at the same temperature. Repeated heating and 30% H2 O2 additions until digest is clear. Add additional HNO3 as needed to maintain a wet digest. After sample digest is clear, removed watch glass and lowered temperature to 80°C. Continued heating until near dryness. Added dilute HNO3 (10%), and deionized water to dissolve digest residue and bring sample to final volume [48-49].
At the beginning of study, soil profile characteristics were observed and recorded by a packet penetrometer (Cl-700A, soil Test Inc., USA). Soil samples were mixed, homogenized and separated into three parts, 1/3 of each samples was air-dried and pass through a 2 mm sieve in order to determine p and k content, pH and electrical conductivity and particle-size distribution. The other 2/3 was passed through a 2 mm sieve without drying and 1/3 of it used to determine heavy metals concentration by Atomic Absorption Spectroscopy (AAS) after digestion with aqua-regia. The samples were analyzed by an Atomic Absorption Spectrophotometer Model AA-6200 (Shimadzu, Japan) using an air-acetylene flame for heavy metals: Chrome, Nickel, Lead and Cadmium, using at least five standard solutions for each metal. All necessary precautions were taken to avoid any possible contamination of the sample as per the AOAC guidelines [18-20].
Vegetable Sampling Method
Aerial parts of Basil in every 3 days in companion of E. amoenum were separated in 30 days and washed and digested by wet method according the standard protocol for measuring Cadmium ,Chrome (III) and (VI) , Cobalt, Nickel and Lead. Bioaccumulation factors (BAF-s) were calculated for heavy metal content of plant parts (mg/kg) / heavy metal content of soil (mg/kg), for each metal [17].
All Basil samples were watered each day by tap water (Tehran tap water). The studied samples were managed by the same light situation and some circumstances in order to be compared with each other due to determine the ability of E. amoenum in adsorbing Lead, Cadmium and Nickel from soil and its potential to avoid transferring heavy metals to coriander and keep safe the eating vegetable .
Physical and chemical properties and concentrations of heavy metals (Cobalt, Chromium, Cadmium, Nickel and Lead,) in soils, before and after adding E. amoenum in the growth period of cultivated Basil were measured in every 3 days. In order to assess amount of heavy metals in the soil samples, heavy metal concentrations in soils of studied vases were determined by atomic absorption spectrophotometer [21-26].
Total dissolved solids (TDS)
The total solid concentration in waste effluent represents the colloidal form and dissolved species. The probable reason for the fluctuation of value of total solid and subsequent the value of dissolved solids due to content collision of these colloidal particles. The rate of collision of aggregated process is also influenced by PH of these effluents [48-49].
Chemical oxygen demand (COD)
The chemical oxygen demand test (COD) determines, the oxygen required for chemical oxidation of organic matter with the help of strong chemical oxidant. The COD is a test which is used to measure pollution of domestic and industrial waste. The waste is measure in terms of equality of oxygen required for oxidation of organic matter to produce CO2 and water. It is a fact that all organic compounds with a few exceptions can be oxidizing agents under the acidic condition. COD test is useful in pinpointing toxic condition and presence of biological resistant substances. For COD determination samples were preserved using H2SO4 and processed for COD determination after the entire sampling operation was complete [49-50].
Biochemical oxygen demand (BOD)
For BOD, 5 samples were immediately processed after Collection for the determination of initial oxygen and incubated at 20 °C for 5 days for the determination of BOD5 [49-50].
Chlorides
Chlorides are generally present in natural water. The presence of chloride in the natural water can be attributed to dissolution of salts deposits discharged of effluent from chemical industries, oil well operations, sewage discharge of effluent from chemical industries, etc. [50].
Heavy metal in Effluent
The heavy metals/metalloid in the effluent samples such as Chromium, Zinc, Copper, lead, cadmium, Tin and Cobalt were analyzed before and after treatment by AAS after digestion of plant materials by AOAC method [48, 50]. The plant samples were washed in deionized water dried (24 hrs at 80°C) immediately to stabilize the tissue and stop enzymatic reactions. After drying, samples were ground to pass a 1.0mm screen using the appropriate Wiley Mill. After grinding, the sample were thoroughly mixed and a 5- to 8-g aliquot withdrawn for analyses and storage33. Weighed 0.5 to 1.0 g of dried (80°C) plant material that has been ground (0.5 to 1.0 mm) and thoroughly homogenized and place in a tall-form beaker or digestion tube. Added 5.0 ml concentrated HNO3 (65 %) and cover beaker with watch glass or place a funnel in the mouth of digestion tube and allow to stand overnight or until frothing subsides. Place covered beaker on hot plate or digestion tube into block digester and heat at 125°C for 1 hour. Removed the digestion tube and allowed cooling. Added 1 to 2 ml 30% H2 O2 and digest at the same temperature. Repeated heating and 30% H2 O2 additions until digest is clear. Add additional HNO3 as needed to maintain a wet digest. After sample digest is clear, removed watch glass and lowered temperature to 80°C. Continued heating until near dryness. Added dilute HNO3 (10%), and deionized water to dissolve digest residue and bring sample to final volume [ 9,53].
Chlorides
Chlorides are generally present in natural water. The presence of chloride in the natural water can be attributed to dissolution of salts deposits discharged of effluent from chemical industries, oil well operations, sewage discharge of effluent from chemical industries, etc [50].
Sulphates
Sulphate in one of the major cation occurring in natural water. Sulphate being a stable, highly oxidized, soluble form of sulphur and which is generally present in natural surface and ground waters. Sulphate itself has never been a limiting factor in aquatic systems. The normal levels of sulphate are more than adequate to meet plants need [50-53].
Statistical Analysis
The values reported here are means of five values. Data were tested at different significant levels using student t-test to measure the variations between the contaminations in soil and coriander parameters before and after treated by E. amoenum. One way analysis of variance (One-ANOVA) was used for data analysis to measure the variations of metal concentrations using SPSS 22.0 software (SPSS Inc, IBM, Chicago, IL).
Results
Chemical composition of the wastewater effluent of studied research laboratories profile in pharmacy faculty before treatment by E. amoenum is shown in the table 1. Data is averages of the effluent profiles.
Table 1: Characteristics of Wastewater from Research pharmaceutical laboratories in Pharmaceutical Sciences Branch, Islamic Azad University, Tehran-Iran before treatment
| Parameters | Concentration Range | Average |
| pH | 5.5 – 9.2 | 7.2 |
| BOD5 at 208C (mg/L) | 1,930–3160 | 2560 |
| COD (mg/L): chemical oxygen demand | 1,200 – 7,000 | 2,600
|
| TSS (mg/L): total suspended solids | 30 – 55 | 40
|
| Total alkalinity as CaCO3(mg/L) | 70 – 1,500 | 750
|
| TVA (mg/L) | 70 – 2,000 | 750
|
| Lead (mg/L) | 0.15 – 1.9 | 1.35 |
| Tin (mg/L) | 0.1 – 1.5 | 0.6 |
| Cadmium (mg/L) | 0.17 – 0.45 | 0.30 |
| Mercury (mg/L) | 0.15 – 0.50 | 0.25
|
| Zinc (mg/L) | 0.05 – 0.15 | 0.09
|
| Cobalt (mg/L) | 0.08 – 0.78 | 0.29
|
| Chromium (mg/L) | 0.1 – 0.6 | 0.3 |
| Chloride (mg/L) | 500 – 1,200 | 900 |
| Sulfide (mg/L) | 2-8 | 5 |
| Nitrate (mg/L) | 80-1200 | 340 |
As compared to BOD, COD was very high which is normal for effluent of such pharmaceutical laboratories. The minimum and maximum values ranged between 1,930–3160 and the averaged values ranged between 1,200 – 7,000 mg/L for the studied effluent.
Wastes from food science and Technology research laboratory had acidic state contributed 50% of the total waste flow at 3600 mm3 /day and had a pH of 0.8. The combined toxicology and food science laboratories waste had a pH of 0.9 (including acidic waste stream), whereas the pH of the waste without acidic waste stream was 8.7.
The combined wastewater had average COD, and BOD values of 2560 mg/L and 2600 mg/L. Some heavy metal contents such as copper and zinc of the wastewater was found to be well below the limits according to IS-3306 (1974). Most of the solids present were in a dissolved form, with practically no suspended solids.
This investigation was carried out to determine the accumulation of heavy metals in E. amoenum in contaminated soils by heavy metals result in food science and technology , chemical and toxicology laboratories’ pharmaceutical effluent and wastewater irrigated soil in the vicinity of sewage treatment plant (STP), Pharmacy Faculty, Tehran.
Results in figures 1,2 and 3 showed significant differences in Cr (III) , Cr(VI) and Cobalt up -taking by plant. The best results for uptake of Nickel, cadmium and chrome was in the soil with pH=3.5 while optimum result of up-taking of lead observed in pH= 4.
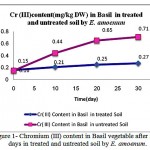 |
Figure 1: Chromium (III) content in Basil vegetable after 30 days in treated and untreated soil by E. amoenum.
|
Plant availability of certain heavy metals depends on soil properties such as soil pH and contain exchange capacity and on the distribution of metals among several soil fractions. The fractionation of Pb, Cr (III) , Cr(VI), Ni, Co and Cd in Basil cultivated control soil and in soils treated by E. amoenum is completely determined due to find out the adsorption ability of heavy metals by E. amoenum in contaminated soil samples. Results showed E. amoenum adsorption for all heavy metals in treated soil were affected significantly by E. amoenum dried content and the adsorbent not only affected contaminated soil and can adsorb Cr (III) , Cr(VI) and Co after 6 days (p<0.001) more than other studied but also adding E. amoenum have reduction and rescue effect in taking up heavy metals especially in bio-adsorbing Cadmium and Nickel more than other heavy metals studied in short time and it keeps edible vegetable safe for eating . In figures 1,2 and 3 the treating contaminated soil trend by E. amoenum indicates that dried plant parts in the soil which is enriched soil by mineral elements and vitamins, it can be consider as a suitable method for rescuing soil by its relatively large ratio of biomass concentration of the contaminant to the soil concentration.
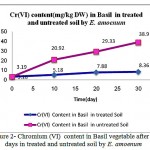 |
Figure 2: Chromium (VI) content in Basil vegetable after 30 days in treated and untreated soil by E. amoenum
|
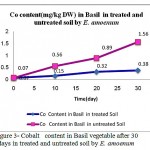 |
Figure 3: Cobalt content in Basil vegetable after 30 days in treated and untreated soil by E. amoenum
|
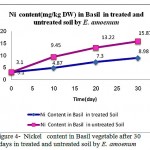 |
Figure 4: Nickel content in Basil vegetable after 30 days in treated and untreated soil by E. amoenum
|
Results in figure 4 showed significant difference in Nickel up -taking by after 20 days , but the potential of taking up Nickel was not as much as Cr (VI), Cr(III) and Co. Moreover, time factor of putting adsorbent in contaminated soil by different heavy metals in the study showed significant (p <0.05) and positive correlation with contents of Pb (r = +85 to r = +90), Cr6+ (r = +90 to r = +92 ), Cr 3+ (r = +93 to r = +95), Ni (r = +41 to r = +43 ) in the contaminated soil and E. amoenum respectively. The amounts of lead adsorbed increased significantly with increase contact time (p<0.005).
Conclusion
The present study focused on adsorption capacity of Cr (VI), Cr(III), Co and Cd , Ni and Pb by E. amoenum was investigated in a batch system by considering the effects of various parameters like contact time, initial concentrations, pH , temperature, absorbent dose. The results of this study revealed that E. amoenum fruit can accumulate high level of Cobalt , Chrome (VI) and (III) in a short time and their uptake rate by vegetable and edible plant is significantly affected by their concentrations in the contaminated soil (p<0.05). A contact time of 15 days by E. amoenum was found to be optimum and 90.6% Cr (VI), 82.3% Cr(III),78.9% Co, 70.3% Pb and 48.6% Ni was taken by adsorbent from contaminated soil while a few amounts of these heavy metals being uptake by edible vegetable – basil. To quantify the occurrence and the distribution of heavy metals, to evaluate their effects, and to prevent them from passing through wastewater collection and treatment systems into soil and ground water bodies represents an urgent task for applied environmental sciences in the coming years. Public acceptance of green technologies is generally higher than that of industrial processes. The responsible organizations should stimulate research to upgrade existing waste water treatment by implementing phytoremediation modules and demonstrating their reliability to the public.
Acknowledgment
Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS) is gratefully acknowledged.
Conflicts of Interest
None of the authors have any conflicts of interest associated with this study.
References
- Seif, H.A.A., Joshi, S.G., Gupta, S.K. Effect of organic load and reactor height on the performance of anaerobic mesophilic and thermophilic fixed film reactors in the treatment of pharmaceutical wastewater. Technol. 1992; 13: 1161–8.
CrossRef - Sudhir Kumar, G., Sunil Kumar,G. Treatment of Pharmaceutical Wastes. Chapter 3, Page 64. Available online : http://ssu.ac.ir/cms/fileadmin/user_upload/Daneshkadaha/dbehdasht/markaz_tahghighat_olom_va_fanavarihaye_zist_mohiti/e_book/pasmand/DK1336_C003.PDF .
- OECD Guidelines for the Testing of chemicals. Dapnia magna, Reproduction Test. OECD 211., Paris, France. 2008.
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use–present knowledge and future challenges.J Environ Manage., 2009; 90: 2354-66.
CrossRef - Daughton, C.G., Ternes, T.A. Pharmaceuticals and personal care products in the environment: agents of subtle change?Environ Health Perspect .,1999; 107 Suppl 6: 907-38.
CrossRef - Kümmerer, K. Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources–a review., 2001; 45: 957-69.
CrossRef - Deshmukh, S.B.; Gadgil, J.S.; Subrahmanyanm, P.V.R. Treatment and disposal of wastewater from synthetic drugs plant (I.D.P.L.), Hyderabad, Part II – Biological treatability. Indian J. Environ. Health ., 1984; 26 :20 –8.
- Murthy, Y.S.; Subbiah, V.; Rao, D.S.; Reddy, R.C.; Kumar, L.S.; Elyas, S.I.; Rama Rao, K.G.; Gadgill, J.S.; Deshmukh, S.B. Treatment and disposal of wastewater from synthetic drugs plant (I.D.P.L.), Hyderabad, Part I – Wastewater characteristics. Indian Environ. Health .,1984;26 (1): 7– 19.
- Howe, R.H.L. Handling wastes from the billion dollar pharmaceuticals Industry. Waste Engng., 1960; 31: 728 –53.
- Lines, G. Liquid wastes from the fermentation industries. J. Water Pollut. Conf. 1968, 655.
- Colovos, G.C., Tinklenberg, N. Land disposal of pharmaceutical manufacturing wastes. Bioeng. , 1962; IV: 153 –60.
- Quane, D.E., Stumpf, M.R. Coal-fired boilers burn waste sludge and odors in an integrated pollution control system. 46th Annual Water Pollution Control Federation Conference.
- Cleveland, O.H. Barker, W.G., Schwarz, D. Engineering processes for waste control. Civil Engng Progress., 1961; 65: 58 – 61.
- Blaine, R.K., Van Lanen, J.H. Application of waste-to-product ratios in fermentation industries. Bioeng. 1962; IV: 129 –38.
CrossRef - Edmondson, K.H. Disposal of antibiotic spent beers by triple effect evaporation. Proceedings of the 8th Industrial Waste Conference, Purdue University, West Lafayette, IN, 1953; 46 – 58.
- Barker, W.G., Stumpf, H.R., Schwarz, D. Unconventional high performance activated sludge treatment of pharmaceutical wastewater. Proceedings of the 28th Industrial Waste Conference, Purdue University, West Lafayette, IN, 1973.
- Gulyas, H., Hemmerling, L., Sekoulov, I. Moglichkeiten zur weitergehenden Entfernung organischer Inhaltsstoffe aus Abwassern der Altolaufbereitung. Z. Wasser Abwasser Forsch. 1991; 24: 253 –7.
- Delaine, J., Gough, D. An evaluation of process for treatment of pharmaceutical effluents. 3rd International Conference on Effluent Treatment from Biochemical Industry, Wheatland: Watford, England, 1980.
- Gulyas, H., von Bismarck, R., Hemmerling, L. Treatment of industrial wastewaters with ozone/hydrogen peroxide. Water Sci. Technol. 1995; 32 (7): 127 –34.
CrossRef - Gupta, S.K., Sharma, R. Biological oxidation of high strength nitrogenous wastewater. Water Res. 1996; 30 (3): 593 – 600.
CrossRef - Ziarati, P., Zolfaghari, M., Azadi, B. The effect of tea residue in promoting phytoremediation of Lavandula angustifoliJ. Plant Anim. Environ. Sci., 2014; 4: 479-86.
- Ziarati, P., Ziarati, N.N. , Nazeri, , Saber-Germi, M. Phytoextraction of heavy metals by two Sorghum spices in treated soil using black tea residue for cleaning-up the contaminated soil. J. Chem., 2015; 31: 317-26.
- Ziarati, P., Iranzad-Asl, , Asgarpanah, J. Companion pelargonium roseum and rosmarinus officinalis in cleaning up contaminated soil by phytoextraction technique the role of companion plants in boosting phytoremediation potential. J. Plant Anim. Environ. Sci., 2014; 4: 424-30.
- Mehrarad, F., Ziarati, P., Mousavi, Z. Removing Heavy Metals from Pharmaceutical Effluent by Pelargonium grandiflorum. Biomedical & Pharmacology Journal ., 2016; 9(1): 151-61 .
CrossRef - Rechinger, K.H. Flora Iranica. No. 48. Graz: Akademishe Druck-u.ver Lagsanstalt; 1967. p. 215.; Hooper D. Useful Plants and Drugs of Iran and Iraq. Chicago, USA: Field Museum of Natural History; 1937. p. 115.
- Wretensjö, I., Svensson, L., Christie, W.W. Gas chromatographic mass spectrometric identification of the fatty acids in borage oil using the picolinyl ester derivatives. J Chromatogr A.1990 ;521: 89–97.
CrossRef - Hooper, D. Useful Plants and Drugs of Iran and Iraq, Field Museum of Natural History, Chicago, Ill, USA. 1937.
- Soltani , A . Encyclopedia of medicinal plants in traditional medicine, Arjmand Publications, Tehran. 2005; 3 (35) .
- Noorhosseini-Niyaki, S. A. , Ashoori-Latmahalleh, D.. Strategies toward Sustainable Development of Echium amoenum in Iran. Journal of Novel Applied Sciences. 2013; 2(8) ; 206-10.
- Akbarinia, A., Keramati Toroghi, M., Hadi Tavatori, M.H. Effect of Irrigation Intervals on Flower Yield of Echium amoenum Mey & Fisch. Pajouhesh & Sazandegi, 2007; 20(3):122-8.
- Akbarzadeh, A. Assemblage and recognition of medicinal plants of Guilan province (Roodbar County). The Role of Medicinal Plants in Sustainable Development Conference. Islamic Azad University, Shabestar branch, Shabestar, Iran. 2008.
- Amin, G.H. Traditional medicinal plants of Iran. Research Deputy Publications of Hygiene Ministry, Islamic Republic of Iran. 1997. pp.230
- Ebrahimi, A. Efficient Factors of Nationally and Internationally Target Markets Recognition and Determination for Pharmaceutics Herbs. 2008.
- The Role of Medicinal Plants in Sustainable Development Conference. Islamic Azad University, Shabestar branch, Shabestar, Iran.
- Ghandali, R., Mirhosseini, S.M. The Evaluation of medicinal plants and by – products, utilization process (pharmaceutical, hygienic, cosmetic, nutrient) in Iran. The Role of Medicinal Plants in Sustainable Development Conference. Islamic Azad University, Shabestar branch, Shabestar, Iran. (In Persian). 2008.
- Ghassemi, N., Sajjadi, S .E,, Ghannadi, A., Shams-Ardakani, M., Mehrabani, M. Volatile Constituents of a Medicinal Plant of Iran, Echium Amoenum Fisch. and Mey. Journal of Daru, 2003; 11(1): 32-3.
- Gholamzadeh, S., Zare, S., Ilkhanipoor, M. Anxiolytic Effect of Echium Amoenum During Different Treatment Courses. Research Journal of Biological Sciences, 2008;3(2): 176-8.
- Mashayekhi, A .N., Farhangi ,A. A., Momeni, M., Alidousti ,S. Inevestigating the Critical Factors Affecting It Application in Governmental Organizations of Iran: A Delphi Survey. Modarres Human Sciences, 2005;42: 191-231.
- Mehrabani ,M., Ghassemi,N., Sajjadi, E., Ghannadi, A., Shams-Ardakani, M. Main Phenolic Compound of Petals of Echium Amoenum Fisch. And C.A. Mey. A Famous Medicinal Plant of Iran. Daru, 2005a; 13: 65 – 9.
- Mehrabani ,M., Shams-Ardakani, M, Ghannadi, A., Ghassemi Dehkordi, N., Sajjadi Jazi, S. E. Production of Rosmarinic Acid in Echium Amoenum Fisch and C.A. Mey. Cell Cultures. Iranian Journal of Pharmaceutical Research. 2005b; 2: 111-5.
- Naderi Hagy, B., Candy, M., Rezaee, M.B. Primory phytochemical investigation of Echium amoenium. Iranian Journal of Medicinal and Aromatic Plants Research., 2004;20(3): 377-83.
- Niroumanesh, A., Saljoughian poor, M., Baharlouyi, A. Importance of medical plants and the rule in Sustainable Development. The Role of Medicinal Plants in Sustainable Development Conference. Islamic Azad University, Shabestar branch, Shabestar, Iran. 2008.
- Noorhosseini-Niyaki S A, Dalivand Z C, Allahyari M S, Sadeghi SM and Noorhosseini Niyaki S F. A Multiple Response Analysis: Effective Strategies Toward Reduce the Gardeners Problems in North of Iran. International Research Journal of Applied and Basic Sciences, 2012; 3 (8): 1646-50.
- Zargari ,A. Medicinal plants. Tehran University Publications. 1996. pp: 510-46.
- 45- Ziarati, P., Kermanshah, A., Moslehishad, M. Adsorption Heavy Metal from Contaminated Water by Modified Shell of Wild Endemic Almonds: Amygdalus lycioides and Amygdalus wendelboi. Biosciences Biotechnology Research Asia, 2015; 12(3): 2451-7.
CrossRef - Standard methods for the examination of water and waste water.22th Edn., APHA,AWWA,WPCF, Washington .D.C.USA. 2012.;
- Ziarati, P., Khoshhal, Z. , Asgarpanah , J., Qomi, M. Contaminations of heavy metals in tea leaves, finished tea products and liqour in Gilan province, Iran. I Farm Allied Sci., 2013; 2: 383-7.
- Makki, F.M. , Ziarati, P. Determination of histamine and heavy metal concentrations in tomato pastes and fresh tomato (Solanum lycopersicum) in Iran. Res. Asia, 2014;11: 537-44.
CrossRef - Fu, W, Franco, A., Trapp, S. Methods for estimating the bioconcentration factor of ionizable organic chemicals. Environ Toxicol Chem., 2009;28: 1372-9.
CrossRef - FAO .1985. Water quality for Agriculture, 1985: Recommendations of the FAO (Food and Agriculture Organization of the United Nations) for the quality of water used for irrigation purposes. http://www.fao.org .
- 1998. AOAC (Association of Official Analytical Chemists). Wet digestion for non –volatile metals in: AOAC official methods of analysis 1998 , 16th edition, 4th revision, vol.1,chapter 9.
- Jones, J. B., Case V. W., Sampling, handling, and analyzing plant tissue samples – Soil testing and plant analysis, SSSA, Inc., Madison, WI, 1990.
- Alimardan, M., Ziarati P., Jafari-moghadam R. Adsorption of Heavy Metal Ions from Contaminated Soil by B. integerrima Barberry. Biomedical and Pharmacology Journal. 2016; 9 (1): 169-75.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





