How to Cite | Publication History | PlumX Article Matrix
Usenkulova Sholpan Zhenisbekovna1, Marat Isakovich Satayev1 and Samonin Vyacheslav Viktorovich2
1M. Auezov South Kazakhstan State University, Kazakhstan, Shymkent.
2St. Petersburg State Technological Institute (Technical University) Russia St. Petersburg.
Corresponding Author E-mail: maratsatayev@mail.ru
DOI : http://dx.doi.org/10.13005/bbra/2272
ABSTRACT: Active carbon, that can be used in the adsorption cleaning of water environments from pollutants of various composition, was produced. Apricot pit shells were charged into the carbonizing stove, where the process was carried out at a temperature of 700 оC with nitrogen purging during 2 hours. Then, the carbonated product was placed into the activation furnace. The thermal activation was carried out in the flow of СO2 and H2O in the mass ratio 85:15 at a temperature of 800 °С during one hour. The paper presents characteristics of the produced active carbon. Moisture content, total porousness, mesopore, micropore and macropore volume, high brightening ability by methylene blue of the active carbon were determined.
KEYWORDS: Active carbon; apricot shells; thermal activation
Download this article as:| Copy the following to cite this article: Zhenisbekovna U. S, Satayev M. I, Viktorovich S. V. Production of Active Carbons from Apricot Pit Shells by Thermal Activation in the Mixture of Carbon Dioxide and Water Vapors. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Zhenisbekovna U. S, Satayev M. I, Viktorovich S. V. Production of Active Carbons from Apricot Pit Shells by Thermal Activation in the Mixture of Carbon Dioxide and Water Vapors. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=16106 |
Introduction
Currently, capabilities for the complex recovery of local waste products and preparation of adsorbents on their base, which can be successfully used in various sectors of the national economy, take a singular value.
Active carbons are produced from different kinds of natural raw material. Active carbons, differing by high mechanical strength, are produced from shells of olive and other fruit pits, as well as coconut, walnut and other nuts [1-6].
Active carbon production analyses from pistachio shells by chemical activation with ZnCl2 and KOH with the following carbonization in N2 and СО2 environment at different temperatures, curing time, heating rate and chemical ratios were carried out [7]. The activation with ZnCl2 results in more developed porous structure, than that with KOH. Analysis of the pistachio shells’ activation with ZnCl2 showed that the most important parameters are chemical agent concentration and ratio. Maximal value of the specific surface in the activation with ZnCl2 was 2024 m2/g. Yield of the active carbon was 47,4 %.
Activation of date palm pits was analyzed in a pipe furnace in the flow of CO2 to evaluate temperature effect and activation time, as well as flow rate on the active carbon quality [8]. Optimal conditions for production of the porous carbon with maximal surface were 971°C (activation temperature), 56 minutes (activation time) and 5,1 cm3/min (CO2 flow rate). At that, the optimal yield is equal to 14,8 % and the specific surface is equal to 666 m2/g. Nitrogen adsorption isotherms showed that the carbon had microporous structure with 1,76 nm mean diameter.
The paper analyzed chemical and physical activation of coconut shell when producing the active carbon [9]. The active carbon was produced by pyrolysis during 6 hours at a temperature of 400°С, then it was activated with the help of chemical and physical processes. The chemical activation was carried out by ammonium bicarbonate solution at 0,5 %, 1 %, 1,5 %, 2 % and 2,5 % concentrations during 24 hours. The physical activation was carried out in a thermal reactor during 2 hours at maximal temperature of 800 °С. Good features of the active carbon were gained at 2,5% concentration of ammonium bicarbonate. At that, the active carbon yield consisted 56,06 % with the following features: 1,95 % of moisture content, 17,7 % of volatile matters, 3,18% of ash content, 79,12 % of tied carbon and 304,8845 mg/g of adsorptive capacity by iodine.
Analyses on production of active biocarbon from Jatropha carcus were carried out [10]. Jatropha carcusseed coats were carbonized at a temperature of 500 оС with 90 % yield. Then the semicoke was activated by sulphanation. pH, ash content, moisture content and adsorptive capacity of the gained active carbon were analyzed. In the activation, at a temperature of 500°С, the ash content was 9,32 %, the moisture content was 2% and рН was 5,80. The active carbon production involved steeping, drying, crushing, screening, carbonization, sulphanation, water washing, drying and storage. Cost estimation of the offered process for the plant, which may process 2500 kg of Jatropha carcus a day for production of 600 kg active carbon a day, was presented.
Active carbon production process from palm waste shells and their adsorptive properties by Cu, Pb, Cr and Cd were analyzed [11]. The optimal specific surface by BET was reached in the following conditions: 30% concentration of phosphoric acid, 500 оС activation temperature during 2 hours curing time. It was found that the optimal specific surface by BET reached 1058 m2/g, and the mean pore diameter was equal to 20,64 nm. The active carbon pore-size distribution was proved with the help of the scanning electron microscopy. Thermal features of the samples were analyzed using thermogravimetric analyzer. The maximal thermal stability was observed up to 600 оС. The adsorptive analyses on the active carbon, treated by 30 % H3PO4 prove its high adsorptive capacity. 100 % adsorbability by Cr, with the following Pb (99,8%), Cd (99,5%) and Cu (25 %) was noted in this investigation.
Analyses on production of active carbon from sugar cane bagasse were carried out [12]. Zinc chloride in various ratios for the bagasse (zinc chloride: bagasse) 1:1; 0,75:1; 0,5:1 and 0,25:1 was used as the activating agent. For the various ratios, pH values consisted 6,82; 6,84; 6,47 and 6,15, ash content values were 38,7; 39,6; 40,8 and 41,6 wt%, specific surface values were 881,9; 801,2; 741,1 and 705,7 m2/g. It is seen by the analyses isotherms that the best correlation of the active carbon was at the ratio 1:1.
Active carbon is adsorbent in industrial water separation and purification [13]. The active carbon adsorptive capacity depends on its porousness and surface chemistry. This survey considers production of the activated carbon from the great number of rich and cheap materials and agricultural wastes, such as sasanqua camellia, bamboo, cherry stones, tea wastes and Paulownia flower. The chemical and physical activation methods and microwave radiation methods are widely used methods, accepted for the active carbon production.
The paper analyzed application of active carbon obtained from olive pits [14, 15]. High-efficiency active carbons with various chemical features were obtained. The active carbon production process involved activation of olive pits in the presence of argon in the interval of temperatures from 700 to 800 °С, and then activation by ZnCl2 and KOH.
This invention is focused on production of active carbon from macadamia [16]. These macadamia nuts contain outer hulls, shell and kernel. The shell is used as the material for the active carbon. The shell is separated from the hull and kernel and then carbonized and activated for the active carbon production.
Effective activation methods of carbon materials from rice hull, apricot pits and walnut-shell were developed [17]. It was established that the best sorbent with mesoporous structure for fusicoccin purification is the walnut-shell.
The regulating of a structure and a pores volume of activated carbons by mechanical, chemical and thermal activations of agricultural wastes, such as apricot kernel shells were studied [18]. The mechanical activation was provided in a grinding mill, in the aqueous medium. The thermal activation was effected in carbon dioxide atmosphere at temperatures of 100-300°С.
Active carbons from wood shavings were produced by physical and chemical activation using potassium hydroxide in the paper [19]. Semi-carbonization was carried out at a temperature of 200 °С during 15 minutes with activation at a temperature of 500 °С during 45 minutes. The active carbon in 20 % impregnation ratio had the highest iodine number 72,39 mg/g and adsorptive capacity by methylene blue 40 mg/g.
Adsorptive properties of carbon sorbents produced from peat by microwave carbonization were studied [20]. The active carbon produced from the peat by the microwave carbonization is characterized by low moisture content and high adsorptive capacity.
There is a method for active carbon production from fruit pit shells [21] by mechanical activation, chemical activation with digestion of the crushed shell in a zinc chloride solution during 1 hour and the following thermal activation in the flow of carbon dioxide during 1 hour. At that, the pit shell mechanical activation was carried out in a hammering mill, where hammers and striking elements are made in the form of a gear polyhedron, at that the surface of rotating discs and chamber walls is made with radial serration. In the chemical activation, zinc chloride was taken with a view of 0,03 g of ZnCl2 per 1 g of the shell. The thermal activation was carried out at a temperature of 470 оС.
The lack of this method is multi-staging of the process and using of chemical reagents, characterized by high cost and corrosive attack on equipment, as the activator.
There is a method for active carbon production from fruit pit shells [22] by digestion of the crushed shell in a zinc chloride solution in 1 % hydrogen chloride, at that the solution is taken with a view of 0,3 g of zinc chloride per 1 g of the shell, the digestion is carried out during 1 hour, then the thermal activation is carried out in the flow of carbon dioxide at a temperature of 700 °С during 2 hours.
The lack of this method is complication of the process, high cost of the used chemical reagents and application of the acid in the process, that negatively affects corrosion stability of equipment.
Material and methods
The apricot pit shell is the heavy tonnage wastes of canning plants for production of fruit cannery products and oil-and-fat plants for processing of pit raw material. Available volume of the raw material in the Central-Asian region, its low price and transportability, good enough adsorptive and physical-chemical properties, as well as effective methods for regulation by the porous structure make possible and economically feasible their use as the adsorbents for water purification.
Research methods, based on the study of separate regularities were chosen to solve the stated problems. Such analytical equipment, as specific surface analyzer (TriStar 3000, Micromeritics) and specific surface analyzer (Sorbi N.4,1, Meta) was used for determination of isotherms of sorption/desorption and porous structure parameter calculation, measurement of porousness by complete adsorption isotherm; high-efficiency liquid chromatograph (VarianProStar) was used for determination of quantitative and qualitative water analysis.
Elemental analysis was carried out using spectrometer (VARIAN 820-MS), photographic images of the porous structure, qualitative and quantitative shell analysis were carried out using scanning electron microscope (JSM-6490LV).
Classical and modern physical-chemical research methods [23-25], allowing gain complete characteristics of the research objects, by which it is possible to estimate the conformational molecular state, properties and presence of various functional groups and adsorptive properties of the adsorbent, were used to study the thermal activation influence on the structural-sorption and physical-chemical characteristics of the apricot pit shell.
The moisture content, % GOST 12597-67 [26]; total porousness by water – GOST 17219-71 [26]; brightening ability by methylene blue – GOST 4453-74 [27]; ash content – GOST 12596-67 [28] of the active carbon were determined. Total pore volume was determined by GOST 17219-71 [29], the active carbon micropore, transition pore and macropore volume was determined by adsorbed standard vapor (benzol) volume, as well as specific surface value by technique presented in the paper [30]. Distribution of the pore volume by sizes was determined by Hg pressing-in method [30], based on measurement of Hg volume in various hydrostatical pressures.
The active carbon production method is carried out as follows. The fruit pit shell is carbonized at a temperature of 700 оС in the inert-gas atmosphere. Then, the carbonized product is placed into the activation furnace retort, where the active carbon production is carried out. The activating agent, i.e. mixture of carbon dioxide and water is delivered into the activation furnace. The process temperature is maintained in the interval of 700-850 оС, the process duration is 1 hour. The carbon is discharged from the activation furnace and crushed to the necessary fraction.
Results and discussion
The research objective is to develop the active carbon production method, which will provide increase in the sorbing pore volume, increase in its adsorptive capacity and load dropping on the major equipment. The objective is achieved by the fact that dioxide carbon and water vapor mixture in the ratio of 85:15, or furnace gases at the activation temperature of 700-850 оС during 1 hour, are used as the activating agent in the active carbon production method.
The active carbon production method differs by low economic costs and high environmental and technological parameters, due to the absence in the activation area aggressive chemical reagents rendering corrosive attack on the equipment and increasing cost of the product. At that, the active carbon owing to use for the activation carbon dioxide and water vapors, characterized by higher oxidizing ability, has high volume of the sorbing pores and high adsorptive activity towards compounds dissolved in water.
Performance of the active carbon production method is illustrated by the following example. The apricot pit shell in amount of 100 g is loaded into the carbonization furnace, where the process is carried out at a temperature of 700 оС with nitrogen inert gas purging during 2 hours. Then, the carbonized product is placed into the activation furnace. The thermal activation is carried out in the flow of СO2 and H2O in the mass ratio of 85:15 at the temperature of 800 °С during 1 hour. Then, the product is discharged from the furnace and weighted. The active carbon weight is equal to 35 g. Table 1 presents characteristics of the active carbon.
Table 1: characteristics of the active carbon
| Moisture content, % | Total porousness, cm3/g | Mesopore volume, cm3/g | Micropore volume, cm3/g | Macropore volume, cm3/g | Brightening ability by methylene blue, % | Ash content, % |
| Offered carbon | ||||||
| 1 | 1,00 | 0,15 | 0,65 | 0,20 | 200 | <6 |
| Available carbon | ||||||
| <6 | 1,25 | 0,55 | 0,60 | 0,10 | <80 | <6 |
The porous structure parameters of the active carbon from the apricot pit shell are presented in Figures 1, 2, 3 and 4. Raise of temperature from 700 оС to 800 оС results in the porous structure maximal development, where the total pore volume is equal to 1 cm3/g. Raise of the activation temperature from 800 оС to 850 оС is followed by decease in the total pore volume to 0,99 cm3/g. Raise of temperature to 800 оС results in decrease in the micropore volume from 0,65 to 0,64 cm3/g, as available resins and hydrocarbons are decomposed with formation of passive carbon, precipitated on the carbon surface, and results in the pore agglomeration.
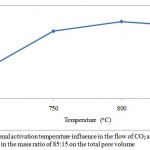 |
Figure 1: Thermal activation temperature influence in the flow of СO2 and H2O in the mass ratio of 85:15 on the total pore volume
|
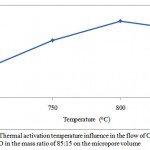 |
Figure 2: Thermal activation temperature influence in the flow of СO2 and H2O in the mass ratio of 85:15 on the micropore volume
|
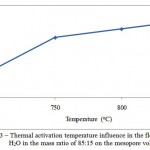 |
Figure 3: Thermal activation temperature influence in the flow of СO2 and H2O in the mass ratio of 85:15 on the mesopore volume
|
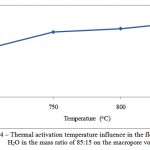 |
Figure 4: Thermal activation temperature influence in the flow of СO2 and H2O in the mass ratio of 85:15 on the macropore volume
|
The elemental analysis using spectrometer VARIAN 820-MS is presented in Figure 5 and Table 2, photographic images of the porous structure using scanning electron microscope JSM-6490LVare presented in Figure 6. By the organic compound elemental analysis results, it was found that the apricot pit shell consists of carbon, hydrogen and nitrogen. The apricot pit shell significantly differs by its anatomical organization from wood substance. Its mechanical tissue consists of stone cells, which have no fibrous form and differs by very thick lignified cover and destroyed protoplast.
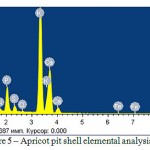 |
Figure 5: Apricot pit shell elemental analysis
|
Table 2: Apricot pit shell elemental-weight ratio
| Element
|
O | Na | Mg | Si | P | S | Cl | K | Ca | Fe |
| Weight % | 50,48 | 1,12 | 5,85 | 0,32 | 4,15 | 1,02 | 0,18 | 27,21 | 13,01 | 0,20 |
 |
Figure 6: Apricot pit shell surface electronic image
|
The offered activation mode provides formation of the developed porous structure with maximal micropore volume in the active carbon, allows gain active carbon with high adsorptive capacity and favors to carry out the active carbon production in the equipment corrosion prevention mode.
Conclusion
The active carbon production method from the apricot pit shell by its carbonization and activation differs by the fact that the mixture of carbon dioxide and water vapors in the ratio of 85:15 at the temperature of 800 оС during 1 hour is used as the activating agent.
In this connection, the actual is to substitute imported raw material by local production wastes with preliminary analysis of their physical-chemical properties and activation of adsorptive capacities in the specified direction, related to the production of very active centers on the surface. Thus, the active carbon production cost significantly reduces.
References
- Branton, P., Bradley, R.H. Effects of active carbon pore size distributions on adsorption of toxic organic compounds. Adsorption, 2011; 17: 293–301.
CrossRef - Anurov, S.A., Anurova, T.V., Klushin, V.N., Mukhin, V.M., Myshkin. V.E. Obtaining of carbonic adsorbents from vegetable waste products. 1- carbonization raw materials. Scientific Research Journal «Investigated in Russia», 2011; 14: 199-211.
- Zabihi, M., Haghighi As.A., Ahmadpour, A. Studies on adsorption of mercury from aqueous solution on activated carbons prepared from walnut shell. Journal of Hazardous Materials, 2010; 174; 251–256.
CrossRef - Park, Y.Su., Lee, J.H., Kim, Y.J., Kim, H.Н. The preparation of new adsorbent by walnut shells. Applied Chemistry, 2006; 10 (№ 2): 645-648.
- Skubiszewska-Zi, J., Sydorchuk, V.V., Gun’ko, V.M. Hydrothermal modification of carbon adsorbents. Adsorption , Online First™, 4 September 2011.
- Lopez-Carzоn, E.J., Moreno-Castilla, G., Guerrero-Ruis, A., Rodrigues-Reinoso, F., Lopez-Gonzalez, J. High temperature adsorption of hydrocarbons by activated carbons prepared from olive stones. Adsorpt. Sci. and Tehnol., 1984; 1 (№ 1): 103-109.
- Hossein Kamandari, Hassan Hashemipour Rafsanjani, Hadi Najjarzadeh, Zahra Eksiri. Influence of process variables on chemically activated carbon from pistachio shell with ZnCl2 and KOH. Research on Chemical Intermediates, 2015; 41,Issue 1: 71-81.
CrossRef - Suresh Kumar Reddy, Ahmed Al Shoaibi, Srinivasakannan, C. Activated carbon from date palm seed: Process optimization using response surface methodology. Waste and Biomass Valorization, 2012; 3,Issue 2: 149-156.
CrossRef - Indah Subadra, Bambang Setiaji, Iqmal Tahir. Activated carbon production from coconut shell with (NH4) HCO3 activator as an adsorbent in virgin coconut oil purification. Prosiding Seminar Nasional DIES ke 50 FMIPA UGM , 2005: 1-8.
- Manyuchi, M. M., Chaukura, N., Chirove, D. Potential for production of activated carbon from jatropha carcus fruit shells. Manyuchi et al., JACSI, 2015; 5(3): 147-152.
- Wan Nik, W.B., Rahman, M.M., Yusof, A.M., Ani, F.N., Che Adnan, C.M. Production of activated carbon from palm oil shell waste and its adsorption characteristics. Proceedings of the 1st International Conference on Natural Resources Engineering & Technology, 2006; Malaysia: 646-654.
- Ajinomoh, C. S., Nurudeen Salahudeen. Production of activated carbon from sugar cane bagasse. Australian Journal of Industry Research. SCIE Journals: 16 – 22.
- Betzy N. Thomas, Soney C. George. Producton of actvated carbon from natural sources. iMedPub Journals, Trends in Green Chemistry, 2015; 1 (№ 1:7): 1 – 5.
- Nawel Spahis, Hacene Mahmoudi. Proposition of a new adsorption refrigeration system using activated carbon prepared from olive stone. J. Int. Environmental Application & Science, 2008; 3 (5): 368-372.
- Spahis, N., Addoun, A., Mahmoudi, H. Study on solar adsorption refrigeration cycle utilizing activated carbon prepared from olive stones. Revue des Energies Renouvelables, 2007; 10 (№ 3): 415 – 420.
- Eiichi Sato. Activated carbon. United States Patent, 1986; № 4,616,001.
- Azat Seitkhan. Development of the technology for fusicoccin preparation using nanocarbon sorbents and study of its biological and cytotoxic activity. Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (PhD), 2015; Almaty: 107 р.
- Marat Isakovich Satayev, Ravshanbek Sultanbekovich Alibekov, Lazzat Mutalovna Satayeva, Omirbek Pernebaiuly Baiysbay, Botagoz Zhaksylykovna Mutaliyeva. Characteristics of activated carbons prepared from apricot kernel shells by mechanical, chemical and thermal activations. Modern Applied Science, 2015; 9 (№6): 104-119.
- Collin G. Joseph, Lawrence Teo Kah Hoong, Faujan B. H. Ahmad. Preparation and characterization of activated carbon derived from rubber wood sawdust (Heaveabrasiliensis): Textural and chemical characterization. Biosciences biotechnology research asia, 2016; 4 (2).
- Natalia K. Kitaeva, Ekaterina A. Bannova, Maria V. Alekseeva, Sergei M. Merkov, Natalia S. Ilicheva. Adsorption properties of carbon sorbents based on carbonized peat. Biosciences biotechnology research asia, 2015; 12(3): 2393-2403.
CrossRef - Satayev, M.I., Mamitova, A.D., Shakirov, B.S., Satayeva, L.M., Turisbaeva, A.K., Smagul, A.T. The process for producing activated carbon Kazakhstan Predritelny paten, 2001; № 10536.
- Satayev, M.I., Altynbekov, F.E., Arginbaev, D.K., Satan, K.I. The process for producing activated carbon. Kazakhstan Predritelny paten, 1999; № 7613.
- Torosyan, V.F. Analiticheskaya khimiya i fiziko-khimicheskiye metody analiza. Prakticheskoye rukovodstvo: uchebno-metodicheskoye posobiye, Tomsk: Izdatel’stvo Tomskogo politekhnicheskogo universiteta, (2010): 195 p.
- Lutsik, V.I., Sobolev, A.Ye., Chursanov, YU.V. Fiziko-khimicheskiye metody analiza. Uchebnoye posobiye, Tver’, (2014): 184 p.
- Mel’chenko, G.G., Yunnikova, N.V. Analiticheskaya khimiya i fiziko-khimicheskiye metody analiza. Kolichestvennyy khimicheskiy analiz. Kemerovskiy tekhnologicheskiy institut pishchevoy promyshlennosti, Kemerovo, (2005): 104 p.
- GOST 12597-67 – Sorbenty. Metod opredeleniya massovoy doli vody v aktivnykh uglyakh i katalizatorakh na ikh osnove.
- GOST 4453-74. Ugol’ aktivnyy osvetlyayushchiy drevesnyy poroshkoobraznyy. tekhnicheskiye usloviya.
- GOST 12596-67. Ugli aktivnyye. Metod opredeleniya massovoy doli zoly.
- GOST 17219-71. Ugli aktivnyye. Metody opredeleniya vremeni zashchitnogo deystviya po benzolu i Por summarnogo ob”yoma po vode..
- Kolyshkin, D.A., Mikhaylova, K.K. Aktivnyye ugli. Leningrad, Khimiya (1972): 56 p.

This work is licensed under a Creative Commons Attribution 4.0 International License.





