How to Cite | Publication History | PlumX Article Matrix
Trianik Widyaningrum1,2, Indro Prastowo*1,4, Masreza Parahadi3 and Angga Dwi Prasetyo3
1Department of Biology Education, Faculty of Teacher Training and Education, Ahmad Dahlan University, Kampus III, Jl. Prof. Dr.Soepomo, Janturan, Yogyakarta 55164, Indonesia.
2Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, Gedung Biologi, Jl. Veteran, Malang 65145, Indonesia.
3Department of Postgraduate Program in Biotechnology, Postgraduate School, Gadjah Mada University, Jl. Teknika Utara, Barek Yogyakarta 55281, Indonesia.
4Nitidipuran Life Science Studio, Jl. Nitidipuran 26, Ngupasan, Gondomanan, Yogyakarta 55122, Indonesia.
DOI : http://dx.doi.org/10.13005/bbra/2274
ABSTRACT: The efforts to produce bioethanol using non-crop raw material are recently developed. Sargassum crassifolium is a promising raw material for biofuel production since containing a high concentration of polysaccharides. Pre-treatment of seaweed at working pressure of 15 Psi, temperature of 121 oC and sulphuric acid concentration of 0.2 M increased the reducing sugars concentration up to 26.68 g/l. The application of cellulase (0.5 g/ml) helped increasing the reducing sugars concentration up to 2.56 times (increasing up to 68.32 g/l). Fermentation using a naturally β-glucosidase producing yeast, Saccharomyces cereviceae (JCM 3012) (0.15 g/ml), produced the final bioethanol conversion up to 90.99%. The activity of β-glucosidase increased up to 20.39 U/ml during fermentation that assisted the degradation of seaweed.
KEYWORDS: Bioethanol; cellulase; seaweed Sargassum crassifolium; Saccharomyces cereviceae JCM 3012; β-glucosidase
Download this article as:| Copy the following to cite this article: Widyaningrum T, Prastowo I, Parahadi M, Prasetyo A. D. Production of Bioethanol from the Hydrolysate of Brown Seaweed (Sargassum Crassifolium) using A Naturally Β-Glucosidase Producing Yeast Saccharomyces Cereviceae JCM 3012. Biosci Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Widyaningrum T, Prastowo I, Parahadi M, Prasetyo A. D. Production of Bioethanol from the Hydrolysate of Brown Seaweed (Sargassum Crassifolium) using A Naturally Β-Glucosidase Producing Yeast Saccharomyces Cereviceae JCM 3012. Biosci Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15656 |
Introduction
Petroleum oil shortage has been a global issue potentially affecting the global economic and also social conditions [1]. Studies on renewable energies (including bioethanol) have long been carried out in order to replace the petroleum oil [2, 3, 4]. Meanwhile, bioethanol production is usually faced to a classical problem associated with “food versus fuel” issues [5]. The usage of non-food raw materials for the production of bioethanol is expected to become a solution to overcome the problem [6]. Morover, the abundance and the avaibility of raw materials is also another concern in the bioethanol production [6].
The availability of brown seaweeds (Sargassum sp.) in some tropical regions is abundant [7, 8, 9, 10]. The use of Sargassum sp as a food ingridient is still relatively limited [7]. Thus, it may potentially overcome the “food versus fuel” problem. On the other hand, seaweeds (including Sargassum sp) can ecologically play an important role as a biosequester that utilizes the atmospheric carbon dioxide for the assimilation as effectively as ligno-cellulosic biomass [11]. However, Sargassum sp have a shorter life cycle as it needs 2 – 3 months to crop in comparison to ligno-cellulosic biomass [12]. The structure of Sargassum sp. is composed of a complex of unique carbohydrate polymers [8, 13]. Degradation of these polymers may provide sugars that present as a precursor for fermentations and other biochemical syntheses [14, 15, 16, 17]. Thus, this bio-resource is potential as a raw material for industrial purposes, including for the biotehanol production.
The aim of this research was to produce a bioethanol from the hydrolysates of Sargassum crassifolium. The production of bioethanol utilizing raw materials containing carbohydrates needs two major steps : (1) hydrolysis of raw materials; and (2) microbial fermentation. The rigid structure of Sargassum crassifolium still leaves problems [8]. Thus, such preparations were carried out which involve the application of pressure, high temperature, strong acid and hydrolytic enzyme. In some cases, the combination of pressure and high temperature is effective in assisting the degradation of ligno-cellulosic materials [8, 18]. Some researchers reported that the sulphuric acid is often used in the degradation of carbohydrate polymers into sugars that shows effective results [8, 17]. Meanwhile, in many cases, the application of cellulase (E.C. 3.2.1.4) helps the degradation of cellulosic carbohydrates (the type of carbohydrate dominating algal cell walls) and produces a high concentration of sugars [8, 14, 15, 17]. Thus, those preparations are expected to hydrolyze algal polysaccharides into sugars. The production of bioethanol was carried out using a β-glucosidase producing species, Saccharomyces cereviceae JCM 3012, which is limited to be reported by other researchers. The presence of β-glucosidase in the fermentation process may further help the cellulolytic degradation since the enzyme degrades smaller polymers of carbohydrates (disaccharides and oligosaccharides) released from the degradation of high molecular cellulose by cellulase [8, 19]. The conversion of high concentration of sugars by the yeast is expected to produce high concentration of bioethanol. The yield of ethanol fermentation was later evaluated.
Materials and Methods
Materials
Brown seaweed (Sargassum crassifolium) was obtained from a local supplier in Gunung Kidul, the Special District of Yogyakarta, Indonesia. Commercial cellulase (with an activity of 30 U/g), available cellulose, 3,5-dinitrosalicylic acid, phenol, sodium sulphite, sodium hydroxide, potassium sodium tartrate, sulphuric acid, acetic acid, sodium acetate trihydrate, and casein were obtained from Sigma Co., (St. Louis, MO, USA). Glucose, cellobiose, cellohexose, agar, and dextrose were obtained from Merck KgaA (Darmstad, Germany). Potatoes were obtained from a local supplier. The culture of Saccharomyces cereviceae (JCM 3012) was obtained from The Food & Nutrition Culture Collection (FNCC), Food and Nutrition Centre, Gadjah Mada University.
Physical and Chemical Preparation of Seaweed
The fresh Sargassum crassifolium was firstly washed to clean the debris. About 10 g of Sargassum crassifolium was added into 100 ml destiled water. Concentrations of sulphuric acid (0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 M) were added into the mixture. The mixture were incubated in a pressurized chamber (Labtech LAC-5040 S, South Korea) with various working pressures (5, 10, and 15 Psi), at a temperature of 121 oC for 15 min. The treated eaweeds were later separated from sulphuric acid prior to homogenization in 100 ml de-mineralized water (25 oC) using a homogenizer (Philips 2115 P, The Netherlands) for 10 min. The reducing sugars were later calculated. Degradation products of cellulose were also calculated for both, pre-treated seaweed (Sargassum crassifolium) and untreated seaweed as the control.
Enzymatic Preparation of Seaweed
About 100 g/ml of the physically and chemically treated pulp of Sargassum crassifolium was added into various concentrations of cellulase (0, 0.1, 0.2, 0.3, 0.4 and 0.5 g/ml). The mixture was later incubated at a temperature of 30 oC, pH 5.5, 1500 rpm for 10 min. The reducing sugars and degradation products of cellulose were later calculated.
The Production of Bioethanol
Various cell concentrations (0.05, 0.1 and 0.15 g/ml) of Saccharomyces cereviceae (JCM 3012) was added into 100 g/l of the physically, chemically and enzymatically treated pulp of Sargassum crassifolium. The mixture was adjusted to anaerobic condition and incubated at a temperature of 30 oC, pH 5.5, 200 rpm for various fermentation periods (0, 12, 24, 36 and 48 hours). The conversion of bioethanol from sugars, reducing sugars, CO2 concentration and the activity of β-glucosidase were later calculated.
The Microbial Preparation
The cells of Saccharomyces cereviceae (JCM 3012) was firstly cultured in a 100 ml of CYG (Casein Yeast Glucose) medium and then incubated at a temperature of 30 oC, pH 5.5 for 24 hours. In order to make the stock, the yeast cells were streaked onto a PDA (Potato Dextrose Agar) medium and incubated a temperature of 30 oC, pH 5.5 for 3 days. The cells were later added into the fermentation process.
Analysis
The reducing sugars were determined using a Spectrophotometer (Genesys-20, USA) at 575 nm according to Miller [20], involving the 3,5-dinitrosalicylic acid as the reagent. Degradation products of cellulose were determined according to Girfoglio et al., [21]. Bioethanol production was determined using a microdiffussion principle and then confirmed by gas chromatography [22, 23]. The conversion of bioethanol was determined as the percentage of bioethanol production from reducing sugars [8]. The CO2 concentration were determined as the same amount of the formed bioethanol [8]. The activity of β-glucosidase was determined according to Nosworthy et al., [19] as the release of one glucose molecule from one molecule of cellobiose.
Results and Discussions
Physical and Chemical Preparation of Seaweed
The pre-treatment of Sargassum crassifolium at working pressures of 15 Psi, temperature of 121 oC and sulphuric acid concentrations of 0.2 M resulted in the increasing concentration of reducing sugar up to 26.68 g/l (Fig. 1). In general, Fig. 1 shows that the increasing working pressures and sulphuric acid concentrations resulted in the increasing reducing sugar concentrations. Meanwhile, the highest yield was obtained at a sulphuric acid concentration of 0.2 M (at all working pressures) (Fig. 1). It is suggested that the application of high temperature (121 oC) may expand glycosidic bonds linking sugar molecules that evolve seaweed polysaccharides [24]. The expanding bonds are potentially enable other substances from surroundings, including moisture (water) and sulphuric acid, to enter into the bonds [8, 14, 24]. On the other hand, the application of various working pressures (5, 10, and 15 Psi) may force water and sulphuric acid to enter into the bonds [18, 24, 25]. In the bonds, the sulphuric acid (acting as an H+ donor) may disrupt glycosidic bonds by binding to an oxygen molecule linking two molecules of sugars [17, 24, 25, 26]. Furthermore, it creates a sugar/glucan-sulphuric acid intermediate [17, 24, 25, 26]. On the other hand, the water dissociates into H+ and OH– [24]. The hydroxyl group (OH–) of water may later attack the atom C of one of sugar/glucan molecules, while H+ may attack the intermediate and release sugars/glucans and sulphuric acid [17, 24, 25, 26]. The disruption, consequently, resulted in the increasing reducing sugar concentrations (Fig. 1).
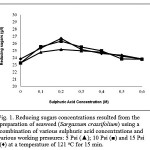 |
Figure 1: Reducing sugars concentrations resulted from the preparation of seaweed (Sargassum crassifolium) using a combination of various sulphuric acid concentrations and various working pressures: 5 Psi (▲); 10 Psi (■) and 15 Psi (♦) at a temperature of 121 oC for 15 min.
|
The increasing sulphuric acid concentrations (0.2 – 0.6 M) resulted in a gradual decrease in reducing sugar concentrations (at all working pressures) (Fig. 1). It is suggested that the excess concentration of sulphuric acid in the reaction system may cause a sugars’ dehydration that lose their functional structures [26, 27]. On the other hand, the combination of physical and chemical treatments dramatically decreased polysaccharides 2.99 times and also released relatively large amounts of oligosaccharides and monosaccharides by 29.85 % and 26.86 %, respectively (Fig. 2).
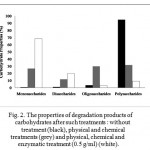 |
Figure 2: The properties of degradation products of carbohydrates after such treatments : without treatment (black), physical and chemical treatments (grey) and physical, chemical and enzymatic treatment (0.5 g/ml) (white).
|
Enzymatic Preparation of Seaweed
The addition of cellulase at various concentrations (0, 0.1, 0.2, 0.3, 0.4 and 0.5 g/ml) into the pulp played a significant role in the hydrolytic reaction, dramatically increasing reducing sugar concentrations up to 2.56 times (increasing up to 68.32 g/l) (Fig. 3). The enzymatic treatment helped to reduce polysaccharides and oligosaccharides (3.5 times and 9.75 times, respectively); and furthermore released large amounts of disaccharides and monosaccharides (1.66 times and 2.56 times, respectively) (Fig. 2). It is suggested that the application of cellulase in the hydrolytic reaction may cleave β-(1,4) glycosidic bonds within polysaccharides (cellulose) and oligosaccharides, and then release simpler sugars, in this case disaccharides and monosaccharides [14, 28].
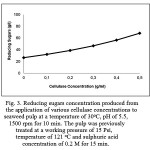 |
Figure 3: Reducing sugars concentration produced from the application of various cellulase concentrations to seaweed pulp at a temperature of 30 oC, pH of 5.5, 1500 rpm for 10 min. The pulp was previously treated at a working pressure of 15 Psi, temperature of 121 oC and sulphuric acid concentration of 0.2 M for 15 min.
|
The Production of Bioethanol
The production of bioethanol is shown in Fig. 4A, 4B and 4C and described as an ethanol conversion from reducing sugars. The final ethanol conversions increased as the increasing of yeast cells concentration (0.05, 0.1 and 0.15 g/ml) (Fig. 4A, 4B and 4C). It is suggested that the increasing concentration of yeast cells increases individual yeasts that naturally convert sugars into ethanol in the fermentation process [8, 29, 30, 31, 32, 33]. Figure 4A, 4B and 4C show a drastic increase in the ethanol conversion (44.22 – 64.28%) in the first 12 hours of the fermentation process (Fig. 4A, 4B and 4C). In this period, yeast cells consumed a high concentration of monosaccharides and converted to bioethanol (Fig. 4A, 4B and 4C) [29, 30, 31, 32, 33]. On the other hand, the activities of β-glucosidase were still low (1.09 – 1.81 U/ml) in the first 12 h of the fermentation process (Fig. 5). It is suggested that the yeasts may still use monosaccharides and may have not secreted the higher amount of β-glucosidase to degrade higher molecular sugars (disaccharides, oligosaccharides, and polysaccharides) in the fermentation process.
 |
Figure 4: Ethanol conversion (♦), carbon dioxide (▲), and reducing sugars concentration (■) during a fermentation process incubated with a β-glucosidase producing yeast Saccharomyces cereviceae (JCM 3012) at various concentrations: 0.05 g/ml (A), 0.1 g/ml (B) and 0.15 g/ml (C) at 30 oC, pH 5.5, 200 rpm for various fermentation periods. Seaweed (Sargassum crassifolium) pulp was previously pre-treated at a working pressure of 15 Psi, temperature of 121 oC, sulphuric acid concentration of 0.2 M for 15 min; followed by the application of cellulase (0.5 g/ml) at a temperature of 30 oC, pH of 5.5 for 10 min.
|
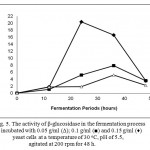 |
Figure 5: The activity of β-glucosidase in the fermentation process incubated with 0.05 g/ml (∆); 0.1 g/ml (■) and 0.15 g/ml (♦) yeast cells at a temperature of 30 oC, pH of 5.5, agitated at 200 rpm for 48 h.
|
A 12 hours fermentation of glucose was separately carried out to determine the glucose conversion to bioethanol and glucose consumption by cells (table 1). The increasing amount of yeast cells (0.05 – 0.15 g/ml) converted glucose to bioethanol (30.21 – 43.92 g/l) and consumed glucose for their cells (25.28 – 34.12%) (table 1). The consumption may consequently impact the amount of the remaining glucose in the reaction system at the end of 12 hours fermentation process (table 1) [29, 30, 31, 32, 33]. Thus, yeasts may show different responses to the remaining glucose concentration in the system. The lowest remaining glucose concentration (1.09 g/l) was obtained from the fermentation of glucose (68.32 g/l) at a yeast incubation of 0,15 g/ml for 12 hours (table 1). On the other hand, disaccharides (19.61 %), oligosaccharides (3.06 %) and polysaccharides (9.01 %) are type of sugars (other than monosaccharides) produced after the enzymatic treatment (Fig. 2). In this condition, yeast may start to use another forms of sugar (disaccharides, oligosaccharides and polysaccharides) and secrete β-glucosidase (E.C. 3.2.1.2.1) to help the degradation. Thus, the activity of β-glucosidase in the fermentation at a yeast incubation of 0.15 g/ml was drastically increasing in a period from 12 to 24 hours (increasing from 1.81 U/ml to 20.39 U/ml) (Fig. 5). As β-glucosidase degraded, yeast consumed and converted sugars to bioethanol. Thus, bioethanol conversion increased gradually, although not as drastically as the conversion in the first 12 h of fermentation. The conversion later culminated at 90.99% at the end of fermentation (Fig. 4C). On the other hand, yeasts consumed less glucose and left higher remaining glucose concentrations in the system in the fermentation of glucose (68.32 g/l) at a yeast incubation of 0.05 and 0.1 g/ml for 12 hours (table 1). Thus, yeast may still consume the remaining glucose and secrete a little amount of β-glucosidase. Fig. 5 shows that the activities of β-glucosidase in the fermentation at a yeast incubation of 0.05 and 0.1 g/ml from 12 to 24 hours were much lower than that of at 0.15 g/ml. The highest β-glucosidase activities in the fermentation at a yeast incubation of 0.05 and 0.1 g/ml were obtained at 36 hours as yeast may start to use another forms of sugar (disaccharides, oligosaccharides and polysaccharides). The ethanol conversions gradually increased and culminated at 62.22% and 73.16% for the yeasts incubation at 0.05 and 0.1 g/ml, respectively (Fig. 4A and 4B).
Table 1: Bioethanol production, the glucose conversion to bioethanol, the glucose consumption by cells, the remaining glucose in the reaction system of different fermentation processes incubated with different concentrations of inoculum at a temperature of 30 oC, pH of 5.5, agitated at 200 rpm for 12 h.
| Concentrations of inoculum | The initial concentration of glucose (g/l) | Bioethanol production (g/l) | The glucose conversion to bioethanol (g/l) | The glucose consumption by cells (g/l) | The glucose consumption by cells (%) | The remaining glucose in the reaction system (g/l) |
| Saccharomyces cereviceae JCM 3012 (0,05 g/ml) | 68,32 | 30,21 | 30,21 | 17,27 | 25,28 | 20,84 |
| Saccharomyces cereviceae JCM 3012 (0,1 g/ml) | 68,32 | 37,24 | 37,24 | 19,19 | 28,09 | 11,89 |
| Saccharomyces cereviceae JCM 3012 (0,15 g/ml) | 68,32 | 43,92 | 43,92 | 23,31 | 34,12 | 1,09 |
On the other hand, the activity of β-glucosidase decreased up to 5.77 times (from 20.39 U/ml to 3.53 U/ml) from 24 to 48 hours in the fermentation at a yeast incubation of 0.15 g/ml. It is suggested that the decrease was presumably caused by a collaborative effect of the reducing concentration of disaccharides in the reaction system and the presence of high concentration of ethanol in the reaction system. The activity of β-glucosidase was calculated based on the conversion of disaccharides to monosaccharides by β-glucosidase. As the reducing concentration of disaccharides in the reaction system, β-glucosidase may naturally start to degrade larger polymers of carbohydrate, such as oligosaccharides and polysaccharides [19, 34]. However, oligosaccharides and polysaccharides are structurally more complex than disaccharides [34]. Thus, β-glucosidase may take a relatively longer period to release monnosaccharides from oligosaccharides or polysaccharides rather than from disaccharides. Furthermore, it may decrease the rate of sugars production from those polymers and also consequently decrease the activity of β-glucosidase [34]. On the other hand, the high accumulation of product (ethanol) in the medium may inhibit metabolic enzymes, including β-glucosidase, by forcing the water within enzymes to diffuse to the outside [4]. The mechanism may further cause β-glucosidase unstable and decrease its activity [4]. Meanwhile, the activities of β-glucosidase at yeast incubation of 0.05 and 0.1 g/ml from 24 hour to 36 hour were increasing and both culminated at 36 h. Although increasing and then culminated at 36 hour, the activities of β-glucosidase at yeast incubation of 0.05 and 0.1 g/ml at 36 hours were much lower than that of at 0.15 g/ml at 36 h (Fig. 5). It is suggested that the accumulation of ethanol may influence the stability of β-glucosidase.
The final bioethanol conversion (90.99%) is slightly higher than that of Borines et al., [8]. The final ethanol conversion (89%) was achieved from a 48 hours fermentation process using Sargassum hydrolysate which was previously prepared using sulphuric acid, heat and in combination with the application of cellulase and β-glucosidase (E.C. 3.2.1.2.1) (Borines et al., 2013). The application of β-glucosidase may provide a higher concentration of monosaccharides which more readily to covert into ethanol by yeasts [8]. There is a principal difference in employing β-glucosidase. Borines et al., [8] added the enzyme at beginning of the reaction. Meanwhile, this research used a natural β-glucosidase producing yeast, Saccharomyces cereviceae (JCM 3012), during the fermentation. The application of microbial cells producing particular enzymes in the production of bioethanol is expected to be more efficient since the pre-treatment and the fermentation may work simultanously [35, 36, 37, 38]. The propagating cells may continuously produce a higher amount of enzymes in the reaction system that may potentially reduce the cost for the additional enzymes in the biomass pre-treatment [35, 36, 37, 38]. On the other hand, this research produced a higher final ethanol conversion (90.99%) than that of in some other works using seaweeds and lignocellulosic materials [25, 29, 31, 32, 33, 39, 40, 41].
Conclusions
The application of working pressure (15 Psi), high temperature (121 oC) in combination with sulphuric acid concentration (0.2 M) in the pre-treatment of Sargassum crassifolium increased the reducing sugars concentration up to 26.68 g/l. The addition of cellulase (0.5 g/ml) into the physically and chemically treated Sargassum crassifolium pulp increased the reducing sugars concentration up to 2.56 times (increasing up to 68.32 g/l). The fermentation using Saccharomyces cereviceae (JCM 3012) (0.15 g/ml) produced the highest bioethanol conversion (90.99%). The highest bioethanol conversion was achived due to the help of β-glucosidase activity that increased up to 20.39 U/ml during fermentation.
Acknowledgements
In this opportunity, authors would like to thank to The Ministry of Research, Technology and Higher Education of Republic Indonesia for funding this research through The 2015 Annual Competitive Research Grant (Hibah Bersaing 2015).
References
- Lutz, C., Lehr, U., Wiebe, K.S. Economic effects of peak oil.Energ Policy. 2012; 48: 829-834.
CrossRef - McEwen, J.T., Atsumi, S. Alternative biofuel production in non-natural hosts.Curr Opin Biotechnol. 2012; 23 (5): 744-750.
CrossRef - Varuvel, E.G., Mrad, N., Tazerout, M., Aloui, F. Experimental analysis of biofuel as an alternative fuel for diesel engines.Appl Energ. 2012; 94, 224-231.
CrossRef - Hidayat, C., Prastowo, I., Hastuti, P., Restiani, R. Effect of ethanol concentrations on rice bran protease activity and ester synthesis during enzymatic synthesis of oleic acid ethyl ester in a fed-batch system using crude rice bran (Oryza sativa) lipase.Biocatal and Biotransfor. 2014; 32 (4), 231-235.
CrossRef - Graham-Rowe Agriculture: Beyond food versus fuel.Nature. 2011; 474(7352), S6-S8.
CrossRef - Limayem, A., Ricke, S.C. Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energ Combust. 2012; 38 (4), 449-467.
CrossRef - Borines, M.G., De Leon, R.L., McHenry, M.P. Bioethanol production from farming non-food macroalgae in Pacific island nations: Chemical constituents, bioethanol yields, and prospective species in the Philippines.Renew Sust Energ Rev. 2011; 15(9), 4432-4435.
CrossRef - Borines, M.G., de Leon, R.L., Cuello, J.L. Bioethanol production from the macroalgae SargassumBioresource Technol. 2013; 138, 22-29.
- Indrani, D.J., Budianto, E. A study of extraction and characterization of alginates obtained from brown macroalgae Sargassum duplicatum and Sargassum crassifolium from Indonesia.Dental Journal (Majalah Kedokteran Gigi). 2013; 46(2), 65-70.
CrossRef - Widowati, I., Puspita, M., Stiger-Pouvreau, V., Bourgougnon, N. Potentiality of Using Spreading Sargassum Species from Indonesia as an Interesting Source of Antibacterial and Radical Scavenging Compounds: A Preliminary Study.International Journal of Marine and Aquatic Resource Conservation and Co-existence. 2014; 1(1), 63-67.
- Kaladharan, P., Veena, S., Vivekanandan, E. Carbon sequestration by a few marine algae: observation and projection.J Mar Biol Assoc India. 2009; 51(1), 107-110.
- Yu, Z., Hu, C., Sun, H., Li, H., Peng, P. Pond culture of seaweed Sargassum hemiphyllum in southern China.Chinese J Oceanol Limnol. 2013; 31, 300-305.
CrossRef - Lee, H.Y., Jung, K.H., Yeon, J.H. Repeated-batch operation of surface-aerated fermentor for bioethanol production from the hydrolysate of seaweed Sargassum sagamianum.J Microbiol Biotechnol. 2011; 21 (3), 323-331.
- Caparrós, C., Lant, N., Smets, J., Cavaco-Paulo, Effects of adsorption properties and mechanical agitation of two detergent cellulases towards cotton cellulose.Biocatal and Biotransfor. 2012; 30 (2), 260-271.
CrossRef - Rabello, G.C., Pirota, R.D.P.B., Barros, G.O.F., Farinas, C.S. Addendum to Issue 1-ENZITEC 2012: Simultaneous biosynthesis of biomass-degrading enzymes using co-cultivation of Aspergillus niger and Trichoderma reesei.Biocatal and Biotransfor. 2014; 32 (4), 236-243.
CrossRef - Silveira, M.H.L., de Siqueira, F.G., Rau, M., Silva, L.D., Moreira, L.R.D.S., Ferreira-Filho, E.X., Andreaus, J. Hydrolysis of sugarcane bagasse with enzyme preparations from Acrophialophora nainiana grown on different carbon sources.Biocatal Biotransfor. 2014; 32 (1), 53-63.
CrossRef - Moutta, R.D.O., Ferreira-Leitão, V.S., Bon, E.P.D.S. Enzymatic hydrolysis of sugarcane bagasse and straw mixtures pretreated with diluted acid.Biocatal Biotransform. 2014; 32(1), 93-100.
CrossRef - Schumacher. , Yanik, J., Sinag, A., Kruse, A. Hydrothermal conversion of seaweed in a batch autoclave. J Supercrit Fluid. 2011; 58 (1), 131 – 135.
CrossRef - Nosworthy, N.J., Kondyurin, A., Bilek, M.M., McKenzie, D. Ion implantation treatment of beads for covalent binding of molecules: Application to bioethanol production using thermophilic beta-glucosidase.Enzyme Microb Tech. 2014; 54, 20-24.
CrossRef - Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar, Chem. 1959; 31,426-428.
- Girfoglio, M., Rossi, M., Cannio, R. Cellulose degradation by Sulfolobus solfataricus requires a cell-anchored endo-β-1-4-glucanase. J Bacteriol. 2012; 194(18), 5091-5100.
CrossRef - Li, H., Ma, M.L., Luo, S., Zhang, R.M., Han, P., Hu, W. Metabolic responses to ethanol in Saccharomyces cerevisiae using a gas chromatography tandem mass spectrometry-based metabolomics approach. Int J Biochem Cell Biol. 2012; 44(7), 1087-1096.
CrossRef - Noriega-Medrano, L.J., Vega-Estrada, J., Ortega-López, J., Ruiz-Medrano, R., Cristiani-Urbina, E., del Carmen Montes-Horcasitas, M. Alternative non-chromatographic method for alcohols determination in Clostridium acetobutylicum fermentations.J Microbiol Methods. 2016; 126, 48-53.
CrossRef - Rinaldi, R., Schüth, F. Acid hydrolysis of cellulose as the entry point into biorefinery schemes.Chem Sus Chem. 2009; 2(12), 1096-1107.
CrossRef - Ge, L., Wang, P., Mou, H. Study on saccharification techniques of seaweed wastes for the transformation of ethanol.Renew Energ. 2011; 36(1), 84-89.
CrossRef - Teong, S.P., Yi, G., Zeng, H., Zhang, The effects of emulsion on sugar dehydration to 5-hydroxymethylfurfural in a biphasic system.Green Chem. 2015; 17 (7), 3751-3755.
CrossRef - Sun, Y., Chen, J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresource Technol. 2002; 83 (1), 1 – 11.
CrossRef - Horn, S.J., Vaaje-Kolstad, G., Westereng, B., Eijsink, V. Novel enzymes for the degradation of cellulose.Biotechnol Biofuels. 2012; 5 (1), 1.
CrossRef - Horn, S.J., Aasen, I.M., Ostgaard, K. Ethanol production from seaweed extract. J Ind Microbiol Biot. 2000; 25 (5), 249-254.
CrossRef - Dodić, S., Popov, S., Dodić, J., Ranković, J., Zavargo, Z., Mučibabić, R.J. Bioethanol production from thick juice as intermediate of sugar beet processing. Biomass Bioenerg. 2009; 33 (5), 822-827.
CrossRef - Jang, J.S., Cho, Y., Jeong, G.T., Kim, S.K. Optimization of saccharification and ethanol production by simultaneous saccharification and fermentation (SSF) from seaweed, Saccharina japonica.Bioproc Biosyst Eng. 2012; 35 (1-2), 11-18.
CrossRef - Cho, Y., Kim, H., Kim, S.K., Bioethanol production from brown seaweed, Undaria pinnatifida, using NaCl acclimated yeast.Bioproc Biosyst Eng. 2013; 36 (6), 713-719.
CrossRef - Kumar, S., Gupta, R., Kumar, G., Sahoo, D., Kuhad, R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach.Bioresource Technol. 2013; 135, 150-156.
CrossRef - Cairns, J.R.K., Mahong, B., Baiya, S., Jeon, J. β-Glucosidases: Multitasking, moonlighting or simply misunderstood?.Plant Sci. 2015; 241, 246-259.
CrossRef - Tang, H., Hou, J., Shen, Y., Xu, L., Yang, H., Fang, X., Bao, High β-glucosidase secretion in Saccharomyces cerevisiae improves the efficiency of cellulase hydrolysis and ethanol production in simultaneous saccharification and fermentation.J Microbiol Biotechnol. 2013; 23 (11), 1577-1585.
CrossRef - Lee, W.H., Nan, H., Kim, H.J., Jin, Y. Simultaneous saccharification and fermentation by engineered Saccharomyces cerevisiae without supplementing extracellular β-glucosidase.J Biotechnol. 2013; 167 (3), 316-322.
CrossRef - Muñoz-Gutiérrez, I., Moss-Acosta, C., Trujillo-Martinez, B., Gosset, G., Martinez, Ag43-mediated display of a thermostable β-glucosidase in Escherichia coli and its use for simultaneous saccharification and fermentation at high temperatures.Microb Cell Fact. 2014; 13(1), 1.
- Saha, B.C., Nichols, N.N., Qureshi, N., Kennedy, G.J., Iten, L.B., Cotta, M. Pilot scale conversion of wheat straw to ethanol via simultaneous saccharification and fermentation.Bioresource Technol. 2015; 175, 17-22.
CrossRef - Jung, Y.H., Kim, I.J., Kim, J.J., Oh, K.K., Han, J.I., Choi, I.G., Kim, K.H. Ethanol production from oil palm trunks treated with aqueous ammonia and cellulase.Bioresource Technol. 2011; 102 (15), 7307-7312.
CrossRef - Velmurugan, R., Muthukumar, K. Utilization of sugarcane bagasse for bioethanol production: sono-assisted acid hydrolysis approach. Bioresource Technol. 2011;102(14), 7119-7123.
CrossRef - Ishola, M.M., Jahandideh, A., Haidarian, B., Brandberg, T., Taherzadeh, M.J. Simultaneous saccharification, filtration and fermentation (SSFF): A novel method for bioethanol production from lignocellulosic biomass.Bioresource Technol. 2013; 133, 68-73.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






