How to Cite | Publication History | PlumX Article Matrix
Sarvesh Kumar Mishra and Shailendra Kumar Srivastava
Department of Biochemistry and Biochemical Engineering, JSBB, SHIATS, Allahabad, India.
Corresponding Author E-mail: sarveshmicro @gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2312
ABSTRACT: Laccase is the model enzymes for multi-copper oxidases can be used in bioremediation, beverage processing, ascorbic acid determination, baking, as a biosensor and to improve food sensory factors. An attempt was made to screen, optimize and production laccase enzyme produced by the consortium of laccase producing Bacillus subtilis. To date, laccases connect mostly been unaided and characterized from flora and fauna and fungi, and unaided fungal laccases are used currently in biotechnological applications. In contrast, tiny is known just approximately bacterial laccases, although recent immediate assume ahead in the combined genome analysis suggests that the enzymes are widespread in bacteria. Since bacterial genetic tools and biotechnological processes are skillfully conventional, therefore developing bacterial laccases would be significantly important. Laccase activity was determined by measuring the oxidation of guaiacol at 530 nm. Laccase activity was maximum when manage at the following conditions, 60 hrs incubation, 30 °C temperature, and pH-5, 2% nitrogen sources, 3 % peptone and beef extract and 2 % carbon sources, glucose and sucrose in the production medium. This research summarizes the distribution of laccases among bacteria, and able to producing maximum laccases at the most favorable conditions
KEYWORDS: laccases; Bacillus subtilis and multi-copper oxidases
Download this article as:| Copy the following to cite this article: Mishra S. K, Srivastava S. K. Production of Extracellular Laccase from Bacterial Strain Bacillus Subtilis MTCC 1039 using Different Parameter. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Mishra S. K, Srivastava S. K. Production of Extracellular Laccase from Bacterial Strain Bacillus Subtilis MTCC 1039 using Different Parameter. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15845 |
Introduction
Laccase has wide substrate specificity towards aromatic compounds containing hydroxyl and amine groups. These enzymes were well-known to catalyze the oxidation of a large range of phenolic compounds and aromatic amines. In fungi, they can be found in ascomycetes, Deuteromycetes and most white-rot basidiomycetes (Baldrian, 2006). One of the advantages of laccases is that they reach not require hydrogen peroxide for substrate oxidation and otherwise, they use oxygen as a non-limiting electron acceptor (Michizoe et al., 2005). Laccases are ubiquitous enzymes present in higher plants, bacteria, fungi, insects and lichens (Riva 2006; Lisov et al., 2007). Due to their sophisticated redox potential as compared to the natural world or bacterial laccases, fungal ones are implicated in several biotechnological applications (Brijwani et al., 2010).
Laccases from bacteria, fungi and plant from the point of view of their structure and function. Bacterial and fungal laccases have a similar structure; their amino acid sequences are quite dissimilar. Bacterial laccases frequently occur as monomers, whereas certain fungal laccases take place as isoenzymes that in general oligomerize to form multimeric complexes (Claus, 2004; Sakurai, 2007). In recent years, bacterial laccases have gained higher concentration for their potential in biodegrading environmentally significant phenolic pollutants due to their relatively elevated production rate, high thermostability, and broad pH range, among others (Held, 2005; Hilden, 2009). Recently some bacterial laccases have also been characterized from Azospirillum lipoforum, Bacillus subtilis, Streptomyces lavendulan, S. cyaneus and Marinomonas mediterranea. A lot of roles for laccases in bacterial systems have been recommended and contain roles in melanin production, spore coat resistance against hydrogen peroxide and UV (Jia et al., 2014).
The application and potential of bacterial laccases for bioremediation applications of bacterial laccases very little are recognized. In generally bacteria tolerate a broader range of habitats and grow faster than fungi (Harms et al., 2011). Moreover, in contrast to fungal laccases, some bacterial laccases can be highly active and much more stable at high temperatures, at high pH as well as at high chloride concentrations (Sharma et al., 2007; Bugg et al. 2011; Dwivedi et al., 2011). The strains Bacillus atrophaeus and Bacillus pumilu produced laccase enzymes can degrade and or modify lignin and contribute to the release of fermentable sugars from lignocellulose (Huang et al., 2013). Laccase activity was highest when operated at the following conditions, 72 h incubation, 40 °C temperature, and pH-7, 2% glucose as carbon source and 2% peptone as the nitrogen source in the manufacturing medium from Pseudomonas aeruginosa (Peter and Vandana 2014).
Material and Method
Bacterial strain
Bacterial strain Bacillus subtilis MTCC 1039 was procured from Microbial Type Culture Collection (MTCC) center, Chandigarh, India. The strain was tested for the purity, morphology, and biochemical characteristics. The strains have been tested for laccase producing ability through plate test method. The ability of the bacterial and fungal strains to produce laccase was visualized according to the method of (Kiiskinen et al., 2004).
Measurement of growth
The growths of bacterial strain was inoculated in nutrient broth and grown at 37 °C and 180 rpm in an orbital shaker. The strain was subcultured @ 1:100 in 50 ml fresh nutrient broth media in 250 ml Erlenmeyer flasks and grown for 12 hrs. Aliquots were withdrawn at hourly intervals and the optical densities were measured using spectrophotometer at 600 nm. The uninoculated media was used as a blank.
Laccase activity
The activity of laccase in vivo determined by spectrophotometric tests using phenolic substrates and by monitoring the colored oxidation products. Laccase activity was determined by measuring the oxidation of guaiacol at 530 nm. The reaction mixture was containing 10 mM guaiacol and 100 mM citrate-phosphate buffer (pH 5.6). Absorbance for blank was measured at 470 nm while that of test samples was measured at 530 nm. Protein concentration was determined by the method of (Lowery et al., 1951) with bovine serum albumin. The following formula was used for determination of enzyme activity.
Optimization of culture conditions for enzyme production
A range of process parameters that move the enzyme production were optimized greater than a broad range. The entire adopted for standardization of process parameters was to examine the effect of an individual parameter and to incorporate it at the standardized level previously standardizing the neighboring-door parameter. The effects of organic and inorganic nitrogen sources, carbon sources, regulate in the period, temperature, pH, was studied.
Effect of incubation period on enzyme production
To find out the effect of incubation period on enzyme production. Fifty ml of nutrient broth (NB) culture media was taken in 250 ml Erlenmeyer flasks. The flasks were sterilized, cooled to room temperature, and inoculated with fresh bacterial culture of Bacillus subtilis culture was incubated at 120 rpm at different time intervals, namely 24, 48, 60, 72, 96, 120 and 144 hrs, respectively at 30 °C. This culture was used as inoculums for laccase production studies. The contents of the flasks were centrifuged at 10000 rpm for 10 min at 4 °C and the supernatant was used to assay the enzyme activity at 450 nm. Laccase activity was assayed using the procedure described previously. The sample which is showing high activity considered as 100 % activity.
Effect of temperature on enzyme activity
Environmental temperature is a factor to which the biomass is an inescapable subject matter since cell temperature should become equal to the temperature of culture medium. The media inoculated with fresh bacterial culture of Bacillus subtilis culture was incubated at 120 rpm at different temperature 25, 30, 35, 40, 45, and 50 °C respectively for 60 hrs. This culture was used as inoculums for laccase production studies. The contents of the flasks were centrifuged at 10000 rpm for 10 min at 4 °C and the supernatant was used to assay the enzyme activity at 450 nm. The sample which is showing high activity considered as 100 % activity.
Effect of pH on enzyme activity
The power of hydrogen ions on biological actions is linked to their hydrogen ion concentration on enzyme activity. The fresh media subculture using the bacterial culture of Bacillus subtilis culture and pH were adjusted in each of the flasks from 4, 4.5, 5, 6, 7, and 8 (using HCl or NaOH) was incubated at 120 rpm at 30 °C respectively for 60 hrs. The contents of the flasks were centrifuged at 10000 rpm for 10 min at 4 °C and the supernatant was used to assay the enzyme activity at 450 nm.
Effect of carbon and nitrogen sources on enzyme activity
The nature and sum of carbon and nitrogen sources in the culture medium are significant for the growth and construction of laccase by bacterial. The production medium enriched with varying of carbon sources, specifically, glucose, maltose, sucrose, and starch with the final concentrations (2 %) and varying of inorganic and organic nitrogen sources, specifically, ammonium sulphate, sodium nitrate, peptone, and beef extract with the final concentrations (2 %) pH was adjusted 5 incubated at 120 rpm at 30 °C respectively for 60 hrs. The contents of the flasks were centrifuged at 10000 rpm for 10 min at 4 °C and the supernatant was used to assay the enzyme activity at 450 nm. The sample which is showing high activity considered as 100 % activity.
Results and Discussion
Primary screening of the strains was done by plate assay method. On the solid agar media for isolation, the different isolates could be distinguished by their color and morphology (Fig. 1.). The bacterial culture was investigated for the lignolytic enzyme, laccase activity by using guaiacol technique. A simple screening method was followed in organize to detect laccase producing bacteria on solid media containing 0.02% guaiacol as an indicator was put into effect for screening of laccase producing by bacteria, develop an intense reddish brown color in the medium around the bacterial colony region as laccase indicators (Ang et al., 2010). The appearance of the reddish brown zone in the medium resulted from the oxidative polymerization of guaiacol (Mabrouk et al., 2010).
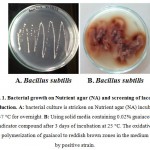 |
Figure 1: Bacterial growth on Nutrient agar (NA) and screening of laccase production. A: bacterial culture is stricken on Nutrient agar (NA) incubated at 37 °C for overnight. B: Using solid media containing 0.02% guaiacol as indicator compound after 3 days of incubation at 25 °C. The oxidative polymerization of guaiacol to reddish brown zones in the medium by positive strain.
|
The strain Bacillus subtilis MTCC 1039 that was capable of producing laccase enzymes was selected as the best strain for future works. The growth pattern of Bacillus subtilis in nutrient broth is shown in fig. 2. The strain Bacillus subtilis was growth pattern showed that this strain is not growth defective.
 |
Figure 2: Bacteria strain does not exhibit defective growth in invitro culture media. Bacillus subtilis strains were grown in broth media. Aliquots were taken out at one-hour intervals and optical density was measured at 600 nm. Data is presented as mean ± S.D. (n = 3).
|
The incubation period of laccase production indicated that the maximum enzyme yield was achieved at 60 hrs of incubation. Among the time, a gradual increase in the enzyme activity was noted at the starting time of incubation period and the maximum enzyme activity was attained at 60 hrs in Fig. 3.
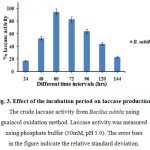 |
Figure 3: Effect of the incubation period on laccase production. The crude laccase activity from Bacillus subtilis using guaiacol oxidation method. Laccase activity was measured using phosphate buffer (50mM, pH 5.0). The error bars in the figure indicate the relative standard deviation.
|
The maximum laccase activity was observed at 30 °C at 60 h of incubation in fig.4. Among the temperature, a gradual increase in the enzyme activity was noted at the starting time of incubation period and the maximum enzyme activity was attained at 30 °C for 60 hrs of incubation in fig. 4. But the production enzyme activity was declined at the higher incubation temperature of 60 °C fig. 4.
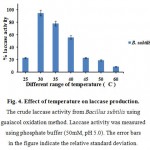 |
Figure 4: Effect of temperature on laccase production. The crude laccase activity from Bacillus subtilis using guaiacol oxidation method. Laccase activity was measured using phosphate buffer (50mM, pH 5.0). The error bars in the figure indicate the relative standard deviation.
|
Hydrogen ions concentration (pH) strongly influences the enzymatic reactions and is receptive to hydrogen ion concentration present in the medium across the cell membrane (Murugesan et al., 2007). Maximum laccase activity was observed at pH 5 for Bacillus subtilis, after a period of 3 hrs fig. 5.
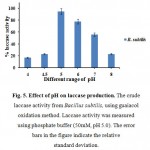 |
Figure 5: Effect of pH on laccase production. The crude laccase activity from Bacillus subtilis, using guaiacol oxidation method. Laccase activity was measured using phosphate buffer (50mM, pH 5.0). The error bars in the figure indicate the relative standard deviation.
|
Nature and type of carbon and nitrogen are the most important factors for any fermentation process (Pandey and Radhakrishnan, 1992). ). In the present study, supplement of the media with special carbon 2 % sources. Among the carbon sources tested, 2 % glucose and sucrose were found to exhibit maximum enzymatic activity then starch and maltose in fig. 6a. Medium containing peptone confirmed the highest laccase activity as enzymes are substrate specific. Peptone is the simplified source of protein and can be voluntarily uptake by the microorganism. Among the tested nitrogen sources, 2 % peptone and 2 % beef extract resulted in higher laccase production fig.6b. Even in the present study, organic nitrogen sources exhibited maximum activity as compared to inorganic sources (Fig. 6a). T. villosa laccase showed improved production using peptone (Morozova et al., 2007). In difference to that, our findings reveal that bacterial strain gives maximum laccase activity with lactose followed by glucose whereas with maltose it does not express laccase activity.
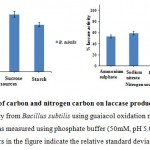 |
Figure 6: Effect of carbon and nitrogen carbon on laccase production. The crude laccase activity from Bacillus subtilis using guaiacol oxidation method. Laccase activity was measured using phosphate buffer (50mM, pH 5.0). The error bars in the figure indicate the relative standard deviation.
|
Conclusion
The laccase production by the Bacillus subtilis strain in nutrient broth was found to be much good than the reported values. The growth and best laccase production of the Bacillus subtilis was favored by acidic pH, 2 % carbon and nitrogen sources at 30 °C for 60 hrs incubation of the medium.
References
- Baldrian, P. (2006). Fungal laccases – occurrence and properties. FEMS Microbiol. Rev. 30, 215-242.
- Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24: 219- 226
- Claus, H. (2004). Laccases: structure, reactions, distribution. Micron 35, 93–96
- Held C., Kandelbauer A., Schroeder M., Cavaco-Paulo A., Guebitz G. (2005) Environ Chem Lett 3: 74–77.
- Jia, H., Lee, F.S. and Farinas, E.T., 2014. Bacillus subtilis Spore Display of Laccase for Evolution under Extreme Conditions of High Concentrations of Organic Solvent. ACS combinatorial science, 16(12), pp.665-669.
- Harms H, Schlosser D, Wick LY (2011) Untapped potential: exploiting fungi in bioremediation of hazardous chemicals. Nat Rev Microbiol 9(3):177–192.
- Sharma P., Goel R., Capalash N. (2007) World J Microbiol Biotechnol 23:823–832.
- Dwivedi U., Singh P., Pandey V., Kumar A.(2011) J Mol Catal B- Enzym 68: 117-128.
- Huang XF, Santhanam N, Badri DV, Hunter WJ Manter DK, Decker SR, Vivanco JM and Reardon KF (2013) Isolation and Characterization of Lignin-Degrading Bacteria from Rainforest Soils, Biotechnol. Bioeng. 30: 30–40.
- Peter JK and Vandana P (2014) Congo red dye decolourization by partially purified laccases from Pseudomonas aeruginosa, Int.J.Curr.Microbiol.App.Sci, 3(9): 105-115.
- Kiiskinen L.-L., K. Kruus, M. Bailey, E.Yl¨osm¨aki, M. Siika-aho,andM. Saloheimo, “Expression ofMelanocarpus albomyces laccase in Trichoderma reesei and characterization of the purifiedenzyme,” Microbiology, vol. 150, no. 9, pp. 3065–3074, 2004.
- Lowery, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J. (1951). The Journal of Biological Chemistry. 193-265.
- Mabrouk, A.M.; Kheiralla, Z.H.; Hamed, E.R. and Youssry, A.A. (2010). Screening of some marine-derived fungal isolates for lignin degrading enzymes (LDEs) production. Agric. Biol. J. N. Am., 2010, 1(4): 591-599.
- Murugesan, A. Dhamija, I.-H. Nam, Y.-M. Kim, and Y.-S. Chang, “Decolourization of reactive black 5 by laccase: optimization by response surface methodology,” Dyes and Pigments, vol. 75, no. 1, pp. 176–184, 2007.
- Pandey, A., Radhakrishnan, S. (1992). Packed bed column bioreactor for production of enzymes. Enzyme Microb. Technol. 14: 486-488.
- Morozova, G. P. Shumakovich, M. A. Gorbacheva, S. V. Shleev, and A. I. Yaropolov, ““Blue” laccases,” Biochemistry (Moscow), vol. 72, no. 10, pp. 1136–1150, 2007.
- Lisov, A.V., Zavarzina, A.G., Zavarzin, A.A. and Leontievsky, A.A., 2007. Laccases produced by lichens of the order Peltigerales. FEMS microbiology letters, 275(1), pp.46-52.
- Michizoe, J., Ichinose, H., Kamiya, N., Maruyama, T. and Goto, M., 2005. Biodegradation of phenolic environmental pollutants by a surfactant–laccase complex in organic media. Journal of bioscience and bioengineering, 99(6), pp.642-647.
- Brijwani, K., Rigdon, A. and Vadlani, P.V., 2010. Fungal laccases: production, function, and applications in food processing. Enzyme Research,2010.
- Sakurai, T. and Kataoka, K., 2007. Basic and applied features of multicopper oxidases, CueO, bilirubin oxidase, and laccase. The Chemical Record, 7(4), pp.220-229.
- Hildén, K., Hakala, T.K. and Lundell, T., 2009. Thermotolerant and thermostable laccases. Biotechnology letters, 31(8), pp.1117-1128.
- Ang TN, Ngoh GC, Chua ASM (2010). A quantitative method for fungal ligninolytic enzyme screening studies. Asia-Pac. J. Chem. Eng. DOI:10.1002/apj.451

This work is licensed under a Creative Commons Attribution 4.0 International License.





