How to Cite | Publication History | PlumX Article Matrix
Al-Ghamdi and A. Al-Muqrin
Princess Nourah University, College of Science, department of Physics, Riyadh, KSA.
Corresponding Author E- mail: hmalghmdi@pnu.edu.sa
DOI : http://dx.doi.org/10.13005/bbra/2273
ABSTRACT: The purpose of this study was to determine the activity concentrations of radium isotopes (226Ra and 228Ra) in groundwaters from some selected wells in Saudi Arabia. These wells are located in different regions of Saudi Arabia. The relationship between the activity concentration of radium and the water chemical compositionin the investigated wells was also investigated.The radium isotopes were measured by gamma spectrometry using high purity germanium detector,after radiochemical separation of the isotopes with ion-exchange chromatography using a strong cation resin. The mean activity concentration of 226Ra was 9.7 pCi/L and for228Ra, it was 9.8 pCi/L. A relatively higher values of radium activities were found in Qaseem, Al Jawf and Tabouk. The 228Ra/226Ra activity ratio had an average value of 1.2. The chemical analyses showed thatgood correlationshave been observed between the radium activities and the total dissolved soilds and dissolved oxygen inthe investigated groundwaters. Most of the analyzed groundwater samples were below the maximum contaminant level of the combined radium (226Ra and 228Ra)proposed by EPA for drinking water. The data obtained from the activity concentrations of the investigatedradium isotopes are comparable to the results reported in literature for radium in the Kingdom of Saudi Arabia, and can be used to establish a radiological baseline radioactivity map for radium radioactivity levels in groundwater of these regions.
KEYWORDS: groundwater; radium; Saudi Arabia; gamma spectrometry; alpha spectrometry; TDS; dissolved oxygen
Download this article as:| Copy the following to cite this article: Al-Ghamdi A, Al-Muqrin A. Radium Activity Levels in Groundwater in Saudi Arabia, and Relationship with Some Water Chemical Constituents. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Al-Ghamdi A, Al-Muqrin A. Radium Activity Levels in Groundwater in Saudi Arabia, and Relationship with Some Water Chemical Constituents. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15929 |
Introduction
Groundater is a very important natural resource related directly to the survival of all living organisms. The concern over exposure of humans to radioactivity is an important driving factor behind the studies of environmental radiation and natural radioactivity levels in groundwaters. There are four naturally-occurring radium isotopes with half-lives ranging from 3.7 days to 1600 years. The 226Ra and 228Ra are the decay products of the insoluble Thorium isotopes [1]. The presence of radium in groundwater is influenced by many factors, such as the occurrence of the radium isotope or its parent thorium nuclide in the aquifer matrix, leaching from the rocks penetrated by groundwater, the alpha recoil, the adsorption/desorption from the surface layer and the water chemical composition [2, 3, 4, 5]. In Saudi Arabia, there are many aquifers within the Saudi Arabian territories differ in lithology, age and extension, and some of them are considered as principal or main aquifers, these aquifers have different concentration of radionuclides. Due to arid to semi-arid conditions prevailed on the Arabian peninsula, the annual groundwater recharge is very low and the stored water within most of these aquifers is old (fossil water), therefore it is in direct contact with rocks and soils for a long time, leading to the change of its hydrochemical characters [6, 7]. The objectives of this study are to determine the activity concentrations of 226Ra and 228Ra in the groundwater from different wells in Saudi Arabia and to elucidate the hydrochemical influences, if any, on the observed radium activities under these conditions.
Methodology
Unless otherwise indicated, all references to water refer to deionized water (DDW).
Sampling
The studied wellsare situated at four different areas. Samples were collected from WadiAl-Dawaser, Al Jawf, Tabouk, Al Qasim and Al Sharqiya, as shown in map 2-1.
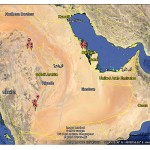 |
Figure |
Water samples (105 samples, 5 liters each) were collected from local wells, and the locations of the sites were precisely recorded using global positioning system (GPS).The water was allowed to run in a continuous flow for a short period to eliminate any contamination from the pipes, and have a representative samples. For radioactivity measurements, sampling was often accompanied by samples treatment (acidification and/or filtration) in order to minimize any interference which could potentially affect the required analysis. Water samples were collected from the continuous flow, filtered with 0.45µ membrane filter, acidified with 11 M HCl at the rate of 10 ml per liter of sample immediately after filtration to avoid the adsorption of radionuclides on the walls of the container and the growth of micro-organisms, and transferred to polyethylene bottles.
Forchemical analysis, the water samples were collected in suitable bottles without the acidification step, and the chemical parameters were monitored with a multi parameter water quality meter, Hachsension 156.
Materials and Apparatus
Radium extractions from water samples were carried out using a strong cation exchange resin, Purolite C-100, Na form, supplied by Veolia Water Co. (Riyadh, Saudi Arabia). Standard reference solutions of 226Ra and 228Ra were supplied by the National Institute of Standardsand Technology (NIST), (SRM 4967A, SRM 4339B). The 133Ba standard solution was supplied by NorthAmerican Technical Services (NATS) (EZ-83879-767). The cation exchange resin was used in a column mode withBioRad Glass Econo columns of 0.9 cm diameter, together withpolypropylene funnels and Teflon end fittings connected withplastic taps.All gamma radioactivity measurements were carried out using a Canberra HPGe coaxial detector (Model GC4020) with relative photo-peak efficiencies of 40% for the 1332 keV line of 60Co. The germanium detector wasconnected to a Digital Spectrum Analysis model DSA-1000. The alpha spectrometric analysis were carried out using a Canberra Alpha Analyst, with a chamber containing a passivated implanted planar silicon (PIPS) detector with an active area of 450 mm2. The efficiency of the detector was calibrated against a standard alpha multi-source (67970-121,Analytics Co.) using the certified activity of the measured radionuclides.Diphonix Resin (50-100 and 100-200 mesh) was supplied from Triskem International, 35170 Bruz, France. All other chemicals used in this study, including KMnO4, isopropanol, ammonium sulphate and different mineral acids were of analytical grade.
Radioanalyses and Measurements
The radium isotopes (226Ra and 228Ra) were determined in the water samples using the procedure described by A.El-Sharkawy et al, 2013 [8]. Four liters of each sample were allowed to pass through the purolite resin packed columns, the resin was transferred to standard counting containersand the containers were tightly sealed for four weeks to allow secular equilibrium between 226Ra, 228Ra and their decay products.The efficiency calibration of the germanium detector for the radium isotopes (Ra-226 and Ra-228) was carried out using standard samples. Known activity resins were prepared by spiking water (DDW) samples with known amounts of 226Ra and 228Ra. The spiked resin samples containing a known amount of the radionuclide of interest were used to provide anidentical matrix with a known activity, and all other conditions were followed typically (flow rate, resin volume, counting time, geometry). The 226Ra activities were determined via its daughters 214Pb and 214Bi through the gamma energy lines 295.22, 351.93 and 609.31 keV. The 228Ra activities were determined through the gamma energy lines of 338.32 and 911.2 keV. The calculated specific activities were basically performed using a comparison method:
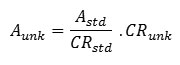
where;
Aunkis the calculated activity of the sample;
Astd is the activity of the standard resin;
CRstd is the counting rate for the standard resin; and
CRunk is the counting rate of the unknown sample.
Errors were propagated due to nuclear counting statistics, tracer and volume.
Quality Assurance
For quality assurance and validation purposes, blank sampleswere prepared in the same manner as the corresponding samples, and measured for background estimation.Reference water samples were determined using the same analysis and measurement protocol, and were compared against their certified values to testthe closeness of the measured samples to its reference values. Also, to evaluate the accuracy of the method, some selected samples were analyzed for 226Ra following an alpha spectrometric method described by S. Nour et al, 2004 [9]. In this approach, 226Ra and 133Ba tracer are co-precipitated with MnO2, dissolved in 2MHCl, loaded into a Diphonix resin column to eliminate other interfering radionuclides and the collected Ra/Ba fraction is then precipitated using BaSO4 micro-precipitation by adding (NH4)2SO4, barium carrier and isopropanol in a tube, mixed well and allowed to stand in an ice bath for 30 min before vacuum filtration[10]. The filter is mounted on a disk, counted by gamma for the 133Ba recovery and the 226Ra is assessed by alpha spectrometry.
Results and Discussion
Radium Isotopes in Groundwater Samples from Alsharqiya Region
The activity concentrations of 226Ra and 228Ra in the groundwater samples from Al Sharqiya region were determined and represented in fig. 3-1.
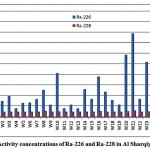 |
Figure 3-1 : Activity concentrations of Ra-226 and Ra-228in Al Sharqiya samples |
The mean activities of 226Ra and 228Ra were 13.7 and 2.5 pCi/L respectively. The 228Ra activities were almost within the low limit of detection (2.3 pCi/L). The mean 228Ra/226Ra activity ratio was 0.3.The 228Ra/226Ra ratio in groundwater may be expected to resemblethe 232Th/238U ratio of the host rock [11].Figures 3-2 and 3-3 present the correlations between the sum of 226Ra and 228Ra, reported as the total radium, and the dissolved oxygen (DO) and oxidation-reduction potential (ORP) respectively.
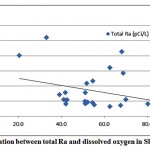 |
Figure 3-2 : Correlation between total Ra and dissolved oxygen in Sharqiya |
As shown in fig. 3-2, no correlation is observed between total Ra and DO in the investigated samples. A weak correlation between the redox potential and total radium was also observed, as shown in fig. 3-3.
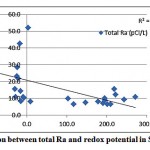 |
Figure 3-3 : Correlation between total Ra and redox potential in Sharqiya |
Radium Isotopes in Groundwater Samples from Al Qasim and Tabouk Region
The mean activities of 226Ra and 228Ra in the Qasim and Tabouk groundwater samples were 10.5 and 15.9 pCi/L respectively, as shown in fig. 3-4
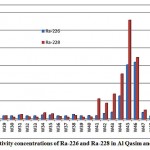 |
Figure 3-4 : Activity concentrations of Ra-226 and Ra-228in Al Qasim and Tabouk |
A good correlation between the redox potential and total radium was also observed, as shown in fig. 3-5.
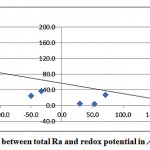 |
Figure 3-5 : Correlation between total Ra and redox potential in Al Qasim and Tabouk |
The total radium in the investigated groundwater samples were found to be strongly associated with the decrease in the dissolved oxygen content of water, as presented in fig. 3-6. It has been reported that more radium is expected in groundwaters with low DO content, where the reducing conditions are more prevailed [5, 12].
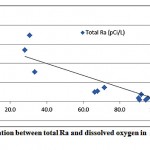 |
Figure 3-6 : Correlation between total Ra and dissolved oxygen inAl Qasim and Tabouk |
Radium Isotopes in Groundwater Samples from Al Jawf Region
The activity concentrations of 226Ra and 228Ra in Al Jawf groundwater samples are represented in fig. 3-7. As shown in figure, the mean activity concentration of Ra-226 is 9.7 pCi/L, ranging from 4.0 to 26.5 pCi/L, while the mean activity concentrationof Ra-228 is 12.8pCi/L, ranging from 2.4 to 33.4pCi/L.
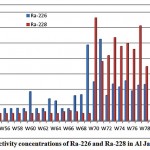 |
Figure 3-7 : Activity concentrations of Ra-226 and Ra-228in Al Jawf region
|
A correlation was observed between total radium and the dissolved oxygen content of the groundwater samples in Al Jawf region, with a correlation coefficient (R2) = 0.46, while a good correlation was found between the combined (226Ra and 228Ra) and the redox potential, as shown in fig. 3-8 and fig. 3-9.
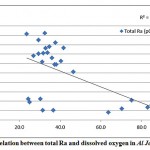 |
Figure 3-8 : Correlation between total Ra and dissolved oxygen in Al Jawf Region |
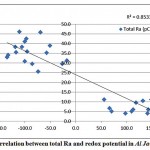 |
Figure 3-9 : Correlation between total Ra and redox potential in Al Jawf Region |
Radium Isotopes in Groundwater Samples from WadiAl-Dawaser Region
Combined radium (226Ra and 228Ra) activity concentrations in groundwater samples from 21 wells were determined, as presented in fig. 3-10.
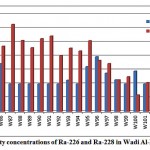 |
Figure 3-10 : Activity concentrations of Ra-226 and Ra-228in WadiAl-Dawaserregion |
The mean activity concentration of Ra-226 is 4.7 pCi/L, ranging from 4.0 to 7.9 pCi/L, while the mean activity concentrationof Ra-228 is 7.9pCi/L, ranging from 2.4 to 12.6pCi/L. From the results of the analyzed groundwater samples, only 228Ra radioisotope presents higher activity than WHO recommendations (about 99% of all the sampledwells). The mean 228Ra/226Ra activity ratio was 1.8. For the relationship between total radium and water chemical constituents, a weak correlation was found between the combined radium and DO, as shown in fig. 3-11.
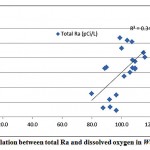 |
Figure 3-11 : Correlation between total Ra and dissolved oxygen in WadiAl-Dawaser |
No correlations were observed between total radium and other water chemical constituents (TDS, ORP, pH).
Conclusion
The activity concentrations of 226Ra and 228Ra were determined in 107 groundwater samples collected from selected wells in Saudi Arabia. Radioactive isotope 228Ra shows highest activity in Al Qasim. Good correlations between some chemical constituents and the activities of the combined radium (226Ra and 228Ra) in the analyzed groundwater samples were observed.The relationship of combined Ra with DO shows that elevated 226Ra and 228Ra activities are strongly inverselyrelated to DO in Al Qasim and Al Jawf. The redox potential has also good correlation with the combined radium in Al Qasim and Al Jawf. No correlations were found between pH, TDS and total radium in the studied groundwater samples. The data obtained may serve as a reference or baseline radiological map for the natural radioactivity levels in these regions in case of any future studies.
Acknowledgment
We express our appreciation to the Deanship of Scientific Research at Princess Nora University for funding this work through the research project 37907/JKD. Also acknowledgment is extended to the chemists and technicians of Technology Experts Co.for their role in the sampling, measurements and analyses of the water samples.
References
- B.L. Dickson, . Radium in groundwater, The Environmental Behavior of Radium, vol. 1. IAEA,Vienna(1990), 335-372.
- P.Vesterbacka. STUK – A213 – 238U-series radionuclides in Finnish grounwater-based drinking water and effective doses – Radiation and Nuclear safety Authority (STUK), Finland(2005), 14-71.
- U.S. Environmental Protection Agency. Understanding Variation in Partition Coefficient, Kd, Values. Volume III: Review of Geochemistry and Available KdValuesfor Americium, Arsenic, Curium, Iodine, Neptunium, Radium, and Technetium. Office of Air and Radiation. July 2004 (EPA402-R-04-002C).
- M.R. Davidson, B.L. Dickson. A porous flow model for steady-state transport of radium in groundwaters, Water. Resour. Res., 22(1986), 34-44.
CrossRef - D.Langmuir, D., Melchior, The geochemistry of Ca, Sr, Ba, and Ra sulfates in some deep brines from the Palo Duro Basin, Texas, Geochim. Cosmochim. Acta, 49(1985), 2423–2432.
CrossRef - H. M. H. Al Sheikh. Water Resources and Development in Saudi Arabia. In Water in the Arabian Peninsula: Problems and Policies. Published by Ithaca Press,2001.
- H. S.Edgell. Aquifers of Saudi Arabia and their Geological Framework. The Arabian Journal for Science and Engineering, Water Resources in the Arabian Peninsula: Part I,1997
- A.El-Sharkawy, Y.Y.Ebaid, W.C.Burnett and S.K.AlDaihan. A rapid and inexpensive method for 226Ra and 228Ra measurements of high TDS groundwaters. ApplRadiatIsot. Vol.77(2013),89-93,.
CrossRef - S. Nour, A. El-Sharkawy, W.C. Burnett and E.P. Horwitz. “Radium-228 Determination of Natural Waters via Concentration on Manganese Dioxide and Separation using Diphonix ion exchange Resin”. Applied Radiation and Isotopes Vol.61(2004), 1173–1178.
CrossRef - S. L. Maxwell, B. K. Culligan. Rapid determination of 226Ra in environmental samples. Journal of Radioanalytical and Nuclear Chemistry, Vol. 293 (1)(2012), 149-156.
CrossRef - S. Krishnaswami and J. Kirk Cochran. Radioactivity in the Environment U-Th Series Nuclides in Aquatic Systems. Volume 13(2008), 1-458.
CrossRef - E., Marie, A., Haquin, G., Zaarur, S., and Ganor, J., 2009, High naturally occurring radioactivity in fossil groundwater from the Middle East: Environmental Science and Technology, v. 43, p. 1769‐1775.

This work is licensed under a Creative Commons Attribution 4.0 International License.





