How to Cite | Publication History | PlumX Article Matrix
Hashemi S. S1, Servatkhah M.1 and Rafati A. R2
1Burn and Wound Healing Research Centre, Shiraz University of Medical Sciences, Shiraz, Iran.
2Division of Pharmacology and Pharmaceutical Chemistry , Sarvestan Branch, Islamic Azad University ,Sarvestan, Iran.
Corresponding Author E-mail: alireza_rafati57@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2321
ABSTRACT: platelet-rich plasma (PRP) is a source of a lot of growth factors and other soluble proteins that can modulate tissue regeneration. Skin fibroblasts play a main role in tissue remodeling and wound repairing. We decided that the human umbilical cord blood platelet-rich plasma (CPRP) affect fibroblast proliferation and cell migration assay. A fibroblast culture was established from a neonatal foreskin. Various concentrations of PRP were prepared from human umbilical cord blood sources and applied to primary human dermal fibroblasts. Cell proliferation was measured by MTT assay. We showed that human umbilical cord blood platelet-rich plasma stimulate the proliferation and migration of dermal fibroblasts and that stimulation by CPRP is dose dependent. human umbilical cord blood platelet-rich plasma can be used to treat chronic skin defects, without triggering an immune response.
KEYWORDS: Human umbilical cord blood; Plasma Rich Platelet; proliferation; migration; human skin fibroblast
Download this article as:| Copy the following to cite this article: Hashemi S. S, Servatkhah M, Rafati A. R. The in Vitro Effect of Different Cord Blood Platelet Rich Plasma Concentrations on Proliferation of Dermal Fibroblasts. Biosci Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Hashemi S. S, Servatkhah M, Rafati A. R. The in Vitro Effect of Different Cord Blood Platelet Rich Plasma Concentrations on Proliferation of Dermal Fibroblasts. Biosci Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15649 |
Introduction
Since skin damage including burns is the most common injuries in the worldwide , in developing countries especially are the major health problems because of severe side effects and limited financial resources [1] so healing of burn’s wound could affect the healing of disease or infection. Wound healing is a complex and dynamic process that includes fast reconstruction of cellular structures and layers of tissue that are damaged [2] this process needs the interaction between cells ,including fibroblasts, smooth muscle cells ,immune cells and factors such as growth factors, hormones, etc[3]. In general, there are four distinct stages in the healing of wounds: hemostasis stage, inflammation stage, proliferation and maturation or remodeling stage in which fibroblast cells play an important role of all [2].
Tissue regeneration begins with degranulation of platelets and clotting leading to the release of various cytokines, clotting factors and inflammatory response. Platelets are naturally stimulating the secretion of growth factors that initiate physiological healing in acute injuries. Platelet-rich plasma (CPRP) contains the increased levels of growth factors (GF) in their biologically determined ratios, including platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-b), insulin-like growth factor-1 (IGF-1), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF), as well as the plasma components fibrin, fibronectin, and vitronectin [4-6]. CPRP plays a significant role in the repair process types of cells, such as osteoblasts, fibroblasts, epithelial cells, endothelial cells and adult mesenchymal stem cells, on a large number of patients [5, 7, 8].
Fibroblasts are critical in supporting normal wound healing, involved in key processes such as breaking down the fibrin clot, creating new extra cellular matrix (ECM) and collagen structures to support the other cells associated with effective wound healing, as well as contracting the wound [9].
Considering the above mentioned and the role of fibroblast cells and growth factors to heal the wounds caused by burns, we decided to examine the CPRP effect on fibroblast proliferation and migration of cells.
Material and method
Cell culture
Human fibroblast cells were isolated from human foreskin and cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), antibiotics such as: streptomycin, penicillin incubated in 5% Co2 incubator at 37 ° C. Culture media was replaced every 3 days. When the cells’ density sticking to the bottom of the flask reached 70 – 80 percent, cells’ passage was done using 25% Trypsin-EDTA solution. Cells from passages 3 and 4 were used.
CPRP Preparation
Umbilical cord blood was collected in lithium heparin–coated collection tubes and initially centrifuged at 350 g for 10 min to separate the red blood cell (RBC) portion from the platelet-rich plasma. Next, the platelets are pelleted by a hard centrifugation of buffy coat plasma at 1600 g for 10 min. Dilutions of the CPRP (5%, 10%, 15%, 20% and 50%) were produced by diluting with standard serum-free media for the various experiments.
Proliferation
Proliferation of the skin fibroblasts was assessed using with total cell number by Colorimetric (crystal violet) proliferation assay. The treatment media used in these studies were serum-free media (negative control), 10% FBS (positive control), 5%, 10%, 15%, 20% and 50% CPRP. As described earlier, all dilutions were prepared with serum-free media.
The viability of cells is determined by MTT assay. This method is based on the succinate dehydrogenase enzyme activity in mitochondria of living cells that turns the yellow MTT solution into the insoluble purple formazan crystals, which can be then dissolved in DMSO and measured with ELISA plate reader. Approximately (1×105) cells were transferred to 96-well plate and incubated for 24 h at 37 ° C, then treated with different concentration of CPRP. The volume of the well was 100 micro liters. Micro plates containing cell extract for 24, were incubated in the same conditions. 10ml solution of MTT (5mg/ml) was added to each well and was incubated for 3 hours. 100 ml DMSO was replaced with incubated MTT medium. Then the optical absorbance was measured at a wavelength of 570 nm with ELISA reader. The viability percentage of cells affected by different concentration of the PRP was calculated by dividing the absorbance of treated wells to the absorbance of control well and then multiplied by 100. The results (mean ± SEM) are expressed using SPSS software [10].
Wound healing assay
Fibroblast cells were added to the six-well plate and allow the plate to fill up the 70% level. After the formation of a cell layer on the bottom of the plate, by using the yellow sampler tip, the bottom of wells was scratched horizontally. Then we rinsed several times with PBS solution for extracting the isolated cells from the medium, using different doses of CPRP all wells were treated and allow cells migrate to gap. Cell migration into the scratch area was analyzed by invert microscope[11].
Statistical analysis
The Mann-Whitney test was used for comparison between groups, with values of p ≤ 0.05 being regarded as significant. Data are presented as means ± SD.
Results
Proliferation
The platelet-rich plasma was prepared by a simple centrifugation process from Umbilical cord blood resulting in a concentration of platelets approximately three to four times the baseline count in cord blood.
The results of the MTT assay are shown in Table 1, with the various treatment media readings presented as ratios compared to the negative control. All components of the CPRP significantly stimulated the growth of fibroblasts when compared to the negative control. Fibroblast growth was enhanced in a dose dependent manner. All fibroblast cultures retained normal morphology.
The proliferation of human dermal fibroblasts peaked on day 4 of culture in the presence of 10% or 20% Umbilical cord platelet-rich plasma and decreased in a dose-dependent manner in the presence of 5% or 50% Umbilical cord platelet-rich plasma. Not only was Cell proliferation observed a little in the serum-free controls, but it markedly increased in the presence of CPRP.
Wound healing assay
The results of the Wound healing assay that are shown as a boxplot in Figure 2. 5%, 10%, 15%, 20% and 50% CPRP had a significant stimulatory effect on the migration of the skin fibroblast cells in comparison with the serum-free media (P < 0.05). 20% CPRP showed a trend toward having a positive effect on migration comparing to the negative control, although statistical significance was not reached.
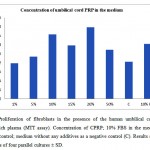 |
Figure1: Proliferation of fibroblasts in the presence of the human umbilical cord blood platelet-rich plasma (MTT assay). Concentration of CPRP; 10% FBS in the medium as a positive control; medium without any additives as a negative control (C). Results shown are the means of four parallel cultures ± SD.
|
 |
Figure 2. Wound healing images after treatment with 20% CPRP (A=0h, B=12h C=24 and D=48h). (Olympus X-10 inverted microscope).
|
Discussion
The wound healing process is a complex mechanism characterized by four distinct phases: hemostasis, inflammation, proliferation and remodelling. All these events are coordinated by cell interactions and soluble growth factors released by various cell type. Platelet-rich plasma is a concentrate of human platelets containing platelet growth factors, as well as plasma components such as fibrin, fibronectin, and vitronectin [12]. It has been postulated that the growth factors within PRP may be secreted in a pulsed fashion, which is the natural mechanism of release, rather than being delivered in a single dose, as is the case when using recombinant growth factors [12]. Platelet Rich Plasma is a biological source of various growth factors which stimulate the proliferation of gingival fibroblast, osteoblast, periodontal ligament fibroblast, stromal stem cell, endothelial cell and enhance healing of ulcers.
In our study we showed that CPRP stimulates the proliferation primary fibroblast cell culture in vitro, because CPRP was associated with releasing a large amounts of PDGF-AB and TGF-beta [13]. The frequency of hematopoietic stem cells and progenitor cells in UCB is greater than the frequency in bone marrow and peripheral blood. CPRP is rich source growth factors including EGF, TGF-beta, IGF-1, Ang-2 and PDGF. CPRP may suppress cytokine release, limit inflammation and thereby promote tissue regeneration. TGF-beta in CPRP stimulated undifferentiated mesenchymal cell proliferation, regulates mitogenic effect. PDGF stimulates chemotaxis and mitogenesis in fibroblast cell [8]. In addition, the use of human UCB as a stem cell source has several advantages over other cell sources, for example, human UCB is abundant and can be obtained with non-invasive methods that raise none of the ethical issues related to other human adult stem cell sources.
In this study, we aimed the quantification of a dose-dependent affected by diluting the CPRP preparation with culture media and compared the response of cells to CPRP preparations. Correlation between CPRP growth factor concentrations and cell function was not an aim, because in the clinical setting, clinicians are not usually aware of the concentration of individual growth factors.
The results of this study did not show a consistent difference in effectiveness between the different CPRP concentrations, but 20% CPRP concentration has strongest effect in human dermal fibroblast proliferation. showed that 20% peripheral blood PRP has strongest effect in human dermal fibroblast, because they could not measure higher concentration with MTT [8]. These results correspond with Krasna with other author’s findings [8, 14] that previously shown the lower concentrations of PRP may have superior effects on cell function. Furthermore, in a rare in vivo study that utilized different PRP concentrations, Weibrich et al found that both high and low PRP concentrations were inferior to intermediate concentrations of PRP in promoting peri-implant wound healing [15].
The results of this study suggest that CPRP could be a very effective enhancer of wound healing. This should Figure 2 Comparison wound healing after treatment with 20% CPRP on dermal fibroblast at several times. CPRP contain plasma components such as fibrin, fibronectin, and vitronectin which are known to be cell adhesion molecules that actively promote cell attachment, as well as forming a matrix scaffold that supports migration and other cellular functions [16].
The results of this study show that CPRP, when applied directly to human skin fibroblasts, can exert a significant effect on cell function of human skin fibroblasts in an in vitro model. Although no adverse effects were noted in this in vitro study, it is still possible that the in vivo use of CPRP could potentially lead to unwanted immunogenic reactions. Hence, the clinical use of CPRP requires further investigation.
Overall, the results of this study show that the use of CPRP promotes wound healing–related fibroblast function, potentially results in faster and more predictable healing of tissues. This may have important wound healing associated with diabetic ulcer regeneration procedures.
CPRP could potentially be utilized as pre-prepared alternatives in clinical procedures and as a nutrient source to support in vitro cell growth in tissue engineering applications.
Acknowledgements
This work was supported by Shiraz University of Medical Sciences [grant number 93-01-63-8177].
References
- Ashkani-Esfahani, S., et al., Enhancement of fibroblast proliferation, vascularization and collagen synthesis in the healing process of third-degree burn wounds by topical arnebia euchroma, a herbal medicine. Galen Medical Journal, 2013. 1(2): p. 53-59.
- Nayak, B. and L.M.P. Pereira, Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complementary and Alternative Medicine, 2006. 6(1): p. 41.
CrossRef - Guo, S. and L.A. DiPietro, Factors affecting wound healing. Journal of dental research, 2010. 89(3): p. 219-229.
CrossRef - Setiawati, E.M., Natural growth factor: platelet rich plasma stimulates proliferation of fibroblast cell culture. Indonesian Journal of Tropical and Infectious Disease, 2010. 1(2): p. 102-104.
CrossRef - Horimizu, M., et al., An improved freeze-dried PRP-coated biodegradable material suitable for connective tissue regenerative therapy. Cryobiology, 2013. 66(3): p. 223-32.
CrossRef - Eppley, B.L., J.E. Woodell, and J. Higgins, Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plastic and reconstructive surgery, 2004. 114(6): p. 1502-1508.
CrossRef - Wroblewski, A.P., H.A. Mejia, and V.J. Wright, Application of platelet-rich plasma to enhance tissue repair. Operative Techniques in Orthopaedics, 2010. 20(2): p. 98-105.
CrossRef - Krasna, M., et al., Platelet gel stimulates proliferation of human dermal fibroblasts in vitro. Acta dermatovenerologica Alpina, Pannonica, et Adriatica, 2007. 16(3): p. 105-110.
- Bainbridge, P., Wound healing and the role of fibroblasts. J Wound Care, 2013. 22(8): p. 407-8, 410-12.
CrossRef - Ebrahimi, M., et al., Appraisal of fibroblast viability in different concentration of glucose as mimicry diabetic condition. Journal of Paramedical Sciences, 2011. 2(4).
- Walter, M., et al., Mesenchymal stem cell-conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Experimental cell research, 2010. 316(7): p. 1271-1281.
CrossRef - Alsousou, J., et al., The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br, 2009. 91(8): p. 987-96.
CrossRef - Carlson, N.E. and R.B. Roach, Jr., Platelet-rich plasma: clinical applications in dentistry. J Am Dent Assoc, 2002. 133(10): p. 1383-6.
CrossRef - Liu, Y., et al., Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Repair Regen, 2002. 10(5): p. 336-40.
CrossRef - Weibrich, G., et al., Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified Curasan kit, with preparations received from a local blood bank. Clin Oral Implants Res, 2003. 14(3): p. 357-62.
CrossRef - Plachokova, A.S., et al., Effect of platelet-rich plasma on bone regeneration in dentistry: a systematic review. Clin Oral Implants Res, 2008. 19(6): p. 539-45.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





