Manuscript accepted on : 12 August 2016
Published online on: --
Plagiarism Check: Yes
To Investigate the Effect of Electromagnetic Radiations on Flavonoids of Lettuce Species
Vishwasini Sharma2 and Leena Parihar1*
1Department of Botany, Seth G.L.Bihani S D Post Graduate College, Sri-Ganganagar, Raj. India.
2Department of Botany, Lovely Professional University, Punjab, India.
Corresponding Author E-mail: leena.parihar@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2324
ABSTRACT: Escalation in use of wireless electronic equipments such as mobile phone, Wi-Fi routers and microwave ovens has rapidly increased the risk of damage in tissue level for all living organisms not only for humans and animals but plants as well. The cell phone use in increasing day by day so we are investigating the positive or negative impact of cell phone radiations on secondary metabolites and morphological parameters of plants. In this current study we are focusing on the effect of electromagnetic radiations on flavonoids of lettuce species at 2G and 3G frequencies for different exposure times. The seeds of lettuce were exposed to cell phone radiations for half hour, 2 hours, 4 hours and 6 hours and later were compared with control. It was concluded that there was reduction in germinating percentage, shoot length, root length and leaf area while fresh weight, dry weight increased. The concentration of flavonoids increased with the increase in the exposure time. As the flavonoids are antioxidant and anti-inflammatory compounds, the use of electromagnetic radiations will help in increased production of such substances and will become the good herbal antioxidant source.
KEYWORDS: electromagnetic radiations; germinating percentage; anti-inflammatory compounds
Download this article as:| Copy the following to cite this article: Sharma V, Parihar L. To Investigate the Effect of Electromagnetic Radiations on Flavonoids of Lettuce Species. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Sharma V, Parihar L. To Investigate the Effect of Electromagnetic Radiations on Flavonoids of Lettuce Species. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=15855 |
Introduction
The readily increase in the rate of cell phones, diverted all the researchers to detect the effect of radio waves on living organisms not only on humans but also on plants. Many studies suggested adverse health symptoms which are caused by mobile phone radiations. Exposure to these mobile phone radiations can cause ill effects; cell damage by formation of reactive oxygen species and ultimately cell death. These radiations are of two types and that are- ionizing and non-ionizing radiations and both of them affect the environment, human and plants. These radiations can be harmful to organisms and result in changes to the natural environment (Hoong, 2003 [8]). The foremost increasing use of mobile phones has affected life of all the organisms and troubled the environment. By using cell phones, the radiations are transferred to the body which causes health problems and they are well known to affect the neurons as they interfere in connectivity of two neurons and that causes many health issues in humans. They cause high blood pressure, fatigue, deafness, migraines and headaches (K Bhargavi, 2013[9]).
Now a days there are various types of cell phone technologies are available for consumers that are 1G, 2G, 3G and 4G but out of them mostly used ones are 2Gand 3G. . The frequency range of 2G is 900-1800 MHz. Radiations that are emitted by the mobile phones are harmful for both plants and animals (Roosli, 2008[16]). 3G operates at the frequency of 2100 MHz. A cell phone has a specific SAR value that is Specific absorption rate (K Bhargavi, 2013[9]).
Lettuce (Lactuca sativa) is an annual herb that belongs to daisy family Asteraceae. Lettuce (Lactuca sativa L.) is one of the most popular among the salad vegetable crops (Whitaker 1969[20]). Nitrogen is required at a very high rate for the growth and development of lettuce. Lettuce is regarded as the first cultivated crop that is being commercialized internationally (Rayyan A et al, 2004[14]). Some compounds that belong to the flavonoids, alkaloids are used as drugs or taken in diet which helps in curing or preventing diseases (Raskin et al, 2002[13]).
All flavonoids have the same basic structure that is a three-ringed molecule. Different flavonoids in a group differ from each other by the number and position of substituents (the hydroxy, methoxy, or sugar groups) (Kumar and Pandey, 2013[10]). These flavonoids possess remarkable biochemical actions like anti-inflammatory, antioxidant, antiallergic, antiviral and anticarcinogenic activities (Hohl et al, 2001[7]; Ren et al, 2003[15]).
We are focusing on the effect of cell phone radiations on Lettuce species which will help us in detecting the effect of 2G and 3G radiations on germination percentage, morphological parameters and in the variation of flavonoid content of control and irradiated seedlings of lettuce species.
Materials and Methods
Material Required
2G and 3G mobile phones, Soxhlet apparatus, Rotary vacuum evaporator, UV-spectrophotometer.
Chemicals Required
Methanol, distilled water, Ferric chloride, Sodium hydroxide, Hydrochloric acid, Lead acetate, Magnesium fillings, Silica gel.
Plant Material
The seeds of Lettuce (Lactuca sativa) were collected from licensed seed store of Phagwara, Punjab. The radiation exposure was given to seeds at different frequencies and different time duration. The first seeds set and second set were exposed to 2G and 3G frequencies for time intervals of 30 minutes, 2 hours, 4 hours and 6 hours respectively (Fig 1). Then the irradiated seeds were sown in pots along with the non-irradiated seeds which were used as control so that we can compare the irradiated and non-irradiated plants.
 |
Figure 1: Radiation exposure to seeds by 2G and 3G cell phones.
|
Morphological Analysis
Five seeds were germinated in petriplates according to the time of exposure for analyzing germination percentage, fresh weight, dry weight; seedling length and for morphological analysis; shoot length, root length and leaf length was measured in 2G and 3G irradiated plants and that was compared with the control ones. After 40-45 days of sowing, lettuce plants were observed for morphological analysis. Washed under tap water then shade dried them for 2-3 days and further drying was done with the help of hot air oven and then grinded to powder form by mechanical grinding. The powdered plant material was used for the extraction of flavonoids which was done with the help of soxhlet apparatus.
Biochemical Analysis
Tests for flavonoids
Some tests were done for the tests of flavonoids and that are given below: (Mahesh et al, 2013[1], Agarwal et al, 2012[3]).
Shinoda Test
To the sample, add 5ml of 95% ethanol and then few pieces (0.5 g) of magnesium turnings were added to this solution followed by few drops of concentrated hydrochloric acid. Observance of pink coloration confirms the presence of flavonoids.
Lead Acetate Test
To the sample, add lead acetate solution which causes formation of yellow precipitate that shows the presence of flavonoid.
Sodium Hydroxide Test
The addition of an increasing amount of sodium hydroxide, the sample containing flavonoids showed yellow coloration which rapidly increased and this decolorized after addition of acid.
Ferric chloride test
To the sample, few drops of ferric chloride solution were added by which formation of blackish red color indicated presence of flavonoids.
Thin Layer Chromatography
Silica gel was applied on TLC Plates and was kept in hot air oven for 3 hours. Chloroform: Methanol with ratio 9:1 was used as the mobile phase. The TLC plates were marked with the sample spot. The solvent front, sample spot was marked, and the plate was finally allowed to dry. The bands that formed were separated by detecting in UV light of 254nm, 366nm and the Colorless components were detected by using visualizing agent, Anisaldehyde spray(A. R Mahesh et al, 2013[1]).
Spectrophotometric Analysis
For determination of total flavonoid content spectrophotometer was used. Quercetin was taken as standard and was prepared by mixing 1 milligram in 1 milliliter of methanol and the optical density was measured at 415 nm by using UV spectrophotometer. The standard curve and the optical density of extracts were compared (Agarwal et al, 2012[4]).
Results and Discussion
Morphological analysis
Table 1: Morphological parameters of lettuce seedlings irradiated at 2G cell phone radiations.
| Time exposure | Germination percentage | Seedling length
(in Cm) |
Fresh weight
(in grams) |
Dry weight
(in grams) |
||||
|
CONTROL |
R1 | R2 | R1 | R2 | R1 | R2 | R1 | R2 |
| 100 % | 100 % | 4.88 | 4.86 | 0.07 | 0.07 | 0.001 | 0.001 | |
|
30 MINS. |
60% |
80% |
4.89 |
4.92 |
0.06 |
0.07 |
0.001 |
0.001 |
|
2 HOURS |
80% |
60% |
4.96 |
5.25 |
0.07 |
0.06 |
0.002 |
0.002 |
|
4 HOURS |
80% |
60% |
5.41 |
5.93 |
0.07 |
0.08 |
0.002 |
0.002 |
|
6 HOURS |
60% |
60% |
6.01 |
5.35 |
0.08 |
0.06 |
0.003 |
0.004 |
The table 1 shows that the germination percentage decreased in 2G irradiated seeds as compare to control ones whereas there was increase in the seedling length, fresh weight and dry weight.
Table 2: Standard Deviation Analysis for Morphological Parameters of lettuce seedlings irradiated at 2G cell phone radiations.
| Exposure time | Germination percentage | Seedling length | Fresh weight |
| CONTROL | 100±0 | 4.87±0.014 | 0.07±0 |
| 30 MINS. | 70±14.142 | 4.905±0.021 | 0.065±0.007 |
| 2 HOURS | 70±14.142 | 5.105±0.205 | 0.065±0.007 |
| 4 HOURS | 70±14.142 | 5.67±0.367 | 0.075±0.007 |
| 6 HOURS | 60±0 | 5.68±0.466 | 0.07±0.014 |
Table 3: Morphological parameters of lettuce seedlings irradiated at 3G cell phone radiations.
| Time exposure | Germination percentage | Seedling length
(in Cm) |
Fresh weight
(in grams) |
Dry weight
(in grams) |
||||
|
CONTROL |
R1 | R2 | R1 | R2 | R1 | R2 | R1 | R2 |
|
100%
|
100% |
4.88 |
4.86
|
0.07 |
0.07
|
0.001
|
0.001
|
|
|
30 MINS. |
80% |
60% |
4.92 |
4.95 |
0.07 |
0.06 |
0.002 |
0.002 |
|
2 HOURS |
60% |
80% |
5.30 |
5.15 |
0.08 |
0.07 |
0.002 |
0.001 |
|
4 HOURS |
60% |
60% |
5.24 |
5.94 |
0.07 |
0.08 |
0.003 |
0.002 |
|
6 HOURS |
60% |
40% |
6.31 |
6.22 |
0.08 |
0.06 |
0.003 |
0.004 |
The table 3 shows that the germination percentage decreased in 3G irradiated seeds as compared to control ones whereas there was increase in seedling length, fresh weight and dry weight.
Table 4: Standard Deviation Analysis for Morphological Parameters of lettuce seedlings irradiated at 3G cell phone radiations.
| Exposure time | Germination percentage | Seedling length | Fresh weight |
| CONTROL | 100±0 | 4.87±0.014 | 0.07±0 |
| 30 MINS. | 70±14.142 | 4.935±0.021 | 0.065±0.007 |
| 2 HOURS | 70±14.142 | 5.225±0.106 | 0.075±0.007 |
| 4 HOURS | 60±0 | 5.59±0.494 | 0.075±0.007 |
| 6 HOURS | 50±14.142 | 6.265±0.636 | 0.07±0.014 |
The reduction in germination percentage was observed more in 3G irradiated seeds as compared to 2G where as there was increase in seedling length, fresh weight, dry weight in 3G irradiated seeds as compared to 2G irradiated seeds.
Morphology Of Plant
The plant morphology can be observed by measuring root length, shoot length and leaf length.
Table 5: Morphological parameters of lettuce plant emerging from seeds irradiated at 2G cell phone radiations.
| Exposure time | Shoot length
(in cm) |
Root length
(in cm) |
Leaf length
(in cm) |
| CONTROL | 4.5 | 7.5 | 16.3 |
| 30 MINS. | 4.5 | 7.4 | 16 |
| 2 HOURS | 4.1 | 7.0 | 15.7 |
| 4 HOURS | 3.9 | 6.6 | 15.5 |
| 6 HOURS | 3.5 | 6.2 | 15.4 |
There was decrease in the shoot length, root length and leaf length in plants emerging from 2G irradiated seeds as compared with those of controlled plants (Table 5).
Table 6: Morphological parameters of lettuce plant emerging from seeds irradiated at 3G cell phone radiations.
| Exposure time | Shoot length
(in cm) |
Root length
(in cm) |
Leaf length
(in cm) |
| CONTROL | 4.5 | 7.5 | 16.3 |
| 30 MINS. | 4.3 | 7.0 | 13 |
| 2 HOURS | 4.0 | 6.5 | 12.7 |
| 4 HOURS | 3.8 | 6.0 | 12.2 |
| 6 HOURS | 3.0 | 5.7 | 11.8 |
There was decrease in the shoot length, root length and leaf length in plants emerging from 3G irradiated seeds as compared with those of controlled plants and also there was more decrease in shoot length, root length and leaf length in plants emerging from 3G irradiated seeds as compared to 2G irradiated ones.
Biochemical Analysis
Table 7: Phytochemical screening
|
TEST |
control |
2G
½ HOUR |
2G
2 HOUR |
2G
4 HOUR |
2G
6 HOUR |
3G
½ HOUR |
3G
2 HOUR |
3G
4 HOUR |
3G
6 HOUR |
| Shinoda test | + | + | + | ++ | ++ | + | ++ | +++ | +++ |
| Lead acetate test | + | + | + | ++ | ++ | + | ++ | +++ | +++ |
| Sodium hydroxide test | + | + | + | ++ | ++ | + | ++ | +++ | +++ |
| Ferric chloride test | + | + | + | ++ | ++ | + | ++ | +++ | +++ |
The table 7 above shows the intensity of flavonoids present with respect to change of colour in each test.
Thin Layer Chromatography
Table 8: Rf value of different samples
| EXPOSURE TIME | Rf VALUE | |
| STANDARD QUERCETIN | 0.93 | 0.93 |
| 2G | 3G | |
| CONTROL | 0.87 | 0.87 |
| 30 MINUTES | 0.87 | 0.89 |
| 2 HOURS | 0.88 | 0.90 |
| 4 HOURS | 0.90 | 0.90 |
| 6 HOURS | 0.91 | 0.92 |
Spectrophotometric Analysis
Fig 2 shows the standard curve of Quercetin which was taken as standard compound
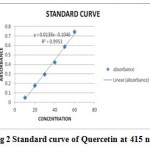 |
Figure 2: Standard curve of Quercetin at 415 nm.
|
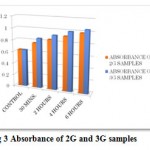 |
Figure 3: Absorbance of 2G and 3G samples.
|
Table 9: Absorbance Of Samples
| EXPOSURE TIME | ABSORBANCE AT 415nm | CONCENTRATION in µg/ml | ||
| 2G | 3G | 2G | 3G | |
| CONTROL | 0.632 | 0.632 | 38.217 | 38.217 |
| 30 MINUTES | 0.765 | 0.845 | 47.855 | 53.652 |
| 2 HOURS | 0.842 | 0.916 | 53.434 | 58.797 |
| 4 HOURS | 0.915 | 0.994 | 58.724 | 62.347 |
| 6 HOURS | 0.984 | 1.04 | 63.724 | 67.782 |
The above table and graph shows that the absorbance increased in 2G and 3G samples as compared to the control ones. This shows that the concentration of flavonoids is increasing with the increase in exposure time of radiations.
Discussion
The effects of radiations on the morphological parameters and secondary metabolites have been discussed above and that showed the increase in the seedling length, fresh weight and dry weight in the seeds that were exposed to radiations as compared to the control ones.
Much work has already accomplished by various workers on different aspects of Lettuce (Boroujerdnia and Ansari, 2007 [4]; Burda and Oleszek, 2001[5]; Coelho et al, 2005[6]). As discussed by Rana and Parihar, 2014[12]; that the radiations cause reduction in the germination percentage, seedling length, fresh weight and dry weight of seedlings. But in current study there was a reduction in germination percentage while other morphological parameters of seedlings. In the previous study the biochemical parameters of the plant increased and in current study there was also an increase in the content of secondary metabolites.
Ragha et al 2011[11] concluded that the plant height and root length was decreased where as biomass percentage was increased with the increase in the frequency, the current study also showed decrease in the root length. Similar results were reported by Sharma and Parihar, 2014 [17] while checking the effects of mobile radiations on nodule formation in the leguminous plants.
Afzal and Mansoor, 2012[2] examined the effect of cell phone radiations on morphology of Mung bean (Vigna radiata) and Wheat (Triticum aestivum) seedlings and concluded that cell phone radiations induce the oxidative stress and caused reduction in growth but increased the antioxidant enzymes activity and like the present study that also showed the reduction in growth whereas increased the flavonoid content in romaine lettuce. Similarly mung bean roots were checked for mobile phone radiation by Sharma et al, 2011[18] whereas Sheikh, 2008[19] checked the resistance of Aloe vera against microwaves.
Conclusion
The present research can be concluded that the cell phones radiations affects the morphological parameters in romaine lettuce and cause increase in the seedling length, fresh weight and dry weight whereas it causes reduction in the germination rate, shoot length, root length and leaf length (Table 1-6). The Rf (retention factor) value was compared with the control and that increased in irradiated samples at different exposure time (Table 8). It also resulted in the increase in concentration of flavonoids which were evaluated by the spectrophotometric analysis and it was observed that the absorbance rate was high in the irradiated samples as compare to the control samples (Fig 3 and Table 9). It can be noted that by exposure to the cell phone radiations there was increase in the concentration of flavonoids (Table 7). This shows that by the electromagnetic radiations the flavonoid content increased and so this indicates cell phones radiations has positive effect on the flavonoid content of romaine lettuce.
Conflict of Interests
“The authors declare that there is no conflict of interests regarding the publication of this article.”
References
- A.R Mahesh, M.K Ranganath and D.R Kumar Harish. 2012. Enrichment of flavonoids from the methanolic extract of Boerhaavia diffusa roots by partitioning technique. Research Journal of Chemical Sciencies, 3(1):43-47
- Afzal Mobin and Mansoor Simeen .2012. Effect of mobile phone radiations on morphological and biochemical parameters of mung bean (Vigna radiate) and wheat (Triticum aestivum) Seedlings. Asian Journal of Agricultural Sciences, 4(2):149-152
- Agarwal Madhu, Kumar Arvind, Upadhyaya Sushant and Ragini Gupta. 2012. Extraction of polyphenol, flavonoid from Emblica officinalis, citrus limon, Cucumis sativus and evaluation of their antioxidant activity. Oriental Journal of Chemistry, 28(2):993-998
CrossRef - Boroujerdnia Maryam and Ansari Alemzadeh Naser. 2007. Effect of different levels of nitrogen fertilizer and cultivars on growth and yield components of Romaine lettuce (Lactuca sativa L.). Middle Eastern and Russian Journal of Plant Science and Biotechnology, 1(2):47-53
- Burda S. and Oleszek W. 2001. Antioxidant and anti- radical activities of flavonoids. J. Agric. Food Chem., 49:2774-2779
CrossRef - Coelho AFS, Gomes EP, Sousa AP, Gloria MBA. 2005. Effect of irrigation level on yield and bioactive amine content of American lettuce. J. Sci. Food. Agric, 85: 1026–1032.
CrossRef - Hohl Ursula, Neubert Barb, Pforte Holger, Schonhof Ilona and Bohm Hartmut. 2001. Flavonoid concentrations in the inner leaves of head lettuce genotypes. Eur Food Res Technol, 213:205-211
CrossRef - Hoong K. Ng. 2003. Non-Ionizing radiations – sources, biological effects, emissions and exposures. International Conference on Non-Ionizing Radiation at UNITEN INCNIR, Electromagnetic genetic fields and our health
- K Bhargavi, Balachandrudu, P Nageswar. 2013. Mobile phone radiation effects on human health. International Journal of Computational Engineering Research, 3(4):196-203
- Kumar Shashank and Pandey K. Abhay. 2013. Chemistry and biological activities of flavonoids : An overview. The Scientific Journal :1-16
- Ragha L, Mishra S, Ramachandran V and Bhatia MS. 2011. Effect of Low Power Microwave Fields on Seeds Germination and Growth Rate. Journal of Electromagnetic Analysis and Applications, 3:165-171
CrossRef - Rana and Parihar. 2014. Effect of mobile phone radiations on protein content of content of soybean seeds. International Journal of Botany and Research, 4(4):1-10
- Raskin I., Ribnicky D.M., Komarnytsky S., Ilic N., Poulev A., Borisjuk N.,Brinker A., Moreno D.A., Ripoll C.and Yakoby N. .2002. Plants and human health in the twenty-first century. Trends in Biotechnology, 20: 522-531
CrossRef - Rayyan A, Kharawish BH, Al-ismail K. 2004. Nitrate content in lettuce heads in relation to plant spacing, nitrogen and irrigation level. J. Sci. Food Agric, 84:931-936
CrossRef - Ren Wenying, Qiao Zhenhua, Wang Hongwei and Zhu Lei, Zhang Li. 2003. Flavonoids: promising anticancer agents. Medicinal Research Reviews, 23(4):519-534
CrossRef - Roosli, M. 2008. Radiofrequency electromagnetic field exposure and non-specific symptoms of ill health: A systematic review. Environmental Research, 107:277-287
CrossRef - Sharma and Parihar. 2014. Effects of mobile radiations on nodule formation in the leguminous plants. Current World Environment, 9(1):145-155
CrossRef - Sharma VP, Singh HP, Batish DR and Kohli RK. 2011. Mobile phone radiation inhibits Vigna radiate (mung bean) Root Growth by Inducing Oxidative Stress. Science Total Environment, 21:5543-5547
- Sheikh A.F.2013. Effect of Microwaves on the resistance of Aloe vera leaves. International Journal of Engineering Research and Applications, 3:242-247
- Whitaker W. Thomas.1969. Salads for everyone: A look at the lettuce plant. Economic Botany, 23(3):261-264
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





