How to Cite | Publication History | PlumX Article Matrix
Akbar Shams*1, Seyed Ali Mortazavi1, Kianosh Khosravi2 and Manochehr Bahmaei3
1Department of Food Science and Technology, Sabzevar Branch, Islamic Azad University, Sabzevar, Iran.
2Research Department of Food Technology, National Nutrition and Food Technology Research Institute, Faculty of Nutrition Sciences and Food Technology, Shahid Beheshti University of Medical Sciences, P. O. Box 19395-4741, Tehran, Iran.
3Department of Food Science and Technology, Islamic Azad University of Tehran, Tehran, Iran.
Corresponding Author's Email: soheil6021@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2375
ABSTRACT: To improve the storage stability, sunflower oil containing poly unsaturated fatty acids (PUFAs) was encapsulated by using ascorbylpalmitate encapsulated with nanoliposomes(APEWN). Ascorbylpalmitate was selected as the wall material because it showeda better antioxidant activity. Microcapsules were prepared and compared with common antioxidants such as BHT and TBHQ.Rancimat method was used for the initial screening of the antioxidant activity. Free fatty acids (FFA), peroxide value (PV), anisidine value and Iodine value of the samples were monitored. The Results showed microen capsulation of ascorbylpalmitate was suitable for preventing sunflower oil from oxidizing in comparison with TBHQ and BHT.
KEYWORDS: lipid oxidation; ascorbylpalmitate; nano- liposome; TBHQ; BHT; sunflower oil
Download this article as:| Copy the following to cite this article: Shams A, Mortazavi S. A, Khosravi K, Bahmaei M. A Comparison Between Ascorbylpalmitateen Capsulated with Nanoliposomeas a Natural Antioxidant And Conventional Antioxidants (TBHQ And BHA) in the Oxidative Stability of Sunflower Oil. Biosci Biotech Res Asia 2016;13(4). |
| Copy the following to cite this URL: Shams A, Mortazavi S. A, Khosravi K, Bahmaei M. A Comparison Between Ascorbylpalmitateen Capsulated with Nanoliposomeas a Natural Antioxidant And Conventional Antioxidants (TBHQ And BHA) in the Oxidative Stability of Sunflower Oil. Biosci Biotech Res Asia 2016;13(4). Available from: https://www.biotech-asia.org/?p=17224 |
Introduction
Sunflower oil is an high-quality edible oil. It is used in cooking, frying, and in the manufacture of margarine and shortening. Sunflower oil is widely used in nutrition as a source of high linoleic acid and vitamin E, compared to other vegetable oils. Furthermore, it is light in taste and appearance. The health benefits associated with polyunsaturated fatty acids (PUFAs) include the anti-inflammatory properties and their positive effects on blood pressure, heart and nervous system function. Sunflower oil has a large content of polyunsaturated fatty acids(Shahidi et al. 1996).
Oil oxidation is an undesirable series of chemical reactions involving oxygen that degrades the quality of oil. The unsaturated long-chain fatty acids present in fats and oils, readily oxidize when exposed to heat, light, and air (Sikwese and Duodu 2007).
It is well known that edible oils are used as cooking media at high temperatures in the presence of oxygen. Therefore, they are subjectedto thermo-oxidation, polymerization, and hydrolysis which eventually cause rancidity in oil, accompanied by off flavor and off-odor, and consequently a decrease in the nutritional quality ofthe product(Siddhurajuand Beeker2003; Minand Lee 1998).
The oil is usuallyexposed to oxidation whichcannot be stopped completely, but there are ways to reduce it. Therefore, attempts should be made to reduce oxidation at each stage of oil manufacture.Different strategies have been used to protect PUFAs from oxidation. A common method of reducing oxidation is the use of a natural (Huber, et al. 2009) or synthetic antioxidant.
Chemically synthesized compounds such as butylatedhydroxyanisole (BHA) and butylatedhydroxytoluene (BHT) are used as antioxidants in oil products. The use of BHA and BTH has proved to have toxic and carcinogeniceffects (Krishnaiah et al. 2010).Therefore, there is an increasing interest in the antioxidant activity of natural compounds. Phospholipids have demonstrated antioxidant activity (Khan andShahidi 2000), and their synergistic effects have been observed in the protection of oil.
Encapsulation is another approach to protecting PUFAs. The encapsulation of PUFA-containing oils increases their oxidative stabilitysignificantly and reduces the undesirable odors of volatile oxidation products. The use of liposomes may be a suitable and promising method to increase the bioavailability and stability of PUFAs and to overcome their drawbacks(Rasti et al. 2012).
The ability of liposomes, the multiple or single phospholipid bilayers surrounding an aqueous medium, as a biocompatible delivery system to help overcome the problem of the reduced solubility of many herbal extractsin lipids is very important(Mozafari et al. 2008; Maherani et al. 2012). Liposomes are an important area in nutritional, biological, and pharmaceutical research, as they are among the most effective carriers for the introduction of various types of bioactive agents into target cells and different parts of non-living systems such as food products (Gregoriadis 2007; Mozafari et al. 2008).
Ascorbylpalmitate is a fat-soluble form of ascorbic acid. This makes ascorbylpalmitateas a very attractive form of vitamin C supplementation.Ascorbylpalmitate can be stored in cell membranes until it is required by the body. A major role of vitamin C is in the production of collagen, a protein that forms the basis of connective tissue – the most abundant tissue in the body (Reynolds 1996).Ascorbylpalmitate is an effective free radical-scavenging antioxidant which promotes skin health and vitality.Ascorbylpalmitate, influencethe cell membrane, provide the antioxidant potential comparable to or even greater than that of vitamin E (Kristl et al. 2003). It also acts synergistically with vitamin E, helping regenerate vitamin E radical in a basic form. For an improved immune system response and advanced antioxidant properties, the advantages of the full health benefits offered in ascorbylPalmitate should be taken(Cort, 1974).McMullen et al. (1991) suggested that the antioxidant effects of ascorbic acid could be more apparent in an oil system if ascorbic acid wasused in combination with other antioxidants such as tocopherols, BHT, and BHA. In fat systems, the use of fat soluble esters of ascorbic acid such as ascorbyl stearate or ascorbylpalmitateis recommended.
In recent years, liposomes have been extensively studied as carrier systems for medical (Khosravi-DaraniandMozafari 2010; Khosravi-Darani et al. 2010; Khosravi-Darani et al.2007; Mortazavi et al. 2007; Mozafari and Khosravi-Darani 2007) and food (Vafabakhsh et al. 2013; Mozafari et al. 2008; Jahadi et al. 2012; 2015; EbrahimiKhoosfi et al. 2014; KhosraviDarani et al.2016; Khanniri et al. 2016) purposes; however, only a few works have been published dealing with the antioxidants encapsulated with nano-liposomes in vegetable oils.
The aim of this work was to evaluate the antioxidant activity of ascorbylpalmitateand to increase the effectiveness and stability of ascorbylpalmitate,as well ascontrol its delivery during storage bynanocapsulationwithnano-liposomes, and to compare them with the known synthetic antioxidants, namelyBHT and TBHQ.
Materials and methods
Materials
Sunflower oil without antioxidants (neutralized, bleached and deodorized) was supplied from Nina, Iran. BHT and BHA were purchased from Sigma Chemical Co. Eicosapentaenoic acid (EPA) andDocosahexanoicacid (DHA) from Novelty,puchongCo Malaysia, soybean phospholipids from the Andre Imcopa, santoCo Brazil, Tetraethoxipropane 1/1/3/3 and Thiobarbituric acid (TBA) from Sigma-Aldrichst.louis, Mo the USE were purchased. Other Chemicals, including methanol, chloroform, n-hexane, ethanol, ferric chloride, and glycine were provided from Merck, Germany.
All tests were carried out at tow temperatures of 25°C and 180 ° C. The samples of oil were formulated at 180 ° C for 24 hours, heated and then prepared at 6 h intervals and packed under the nitrogen condition at -18 ° Cuntil experiments and the oil stability index was measured again.
Methods
Liposome preparation
Empty and encapsulated-PUFA liposomes were prepared according to the procedure previously described by Colas et al. (2007). Briefly, the mixture of the liposomal ingredients, including soybean phospholipids (PLs) (preheated to 30°C) and PUFAs (DHA and EPA; 2:3, w/w) with a mass ratio of 2:0.4, were hydrated by adding deionized water and glycerol (final concentration 2% v/v). The mixture was preheated to 30°C and stirred at 1000 rpm using a hotplate stirrer (MAXIMA Digital, Fisher Scientific, Shah Alam, Malaysia) on a hotplate (IKA_C-MAG HS 10, Petaling Jaya, Malaysia) at 30°C for 60 min. The preparation process was carried out in a six-baffled glass vessel. Compared to the conventional method, in this method, liposomes are prepared by direct hydration and without solving the PLs and FAs in organic solvents. In order to anneal and stabilize the liposomal samples, they were kept at 25°C (ambient temperature) under nitrogen for at least 1 h after preparation.
APEWN, BHT, and TBHQ as the most powerful antioxidants were added to sunflower oilat different concentrations (rapid oxidation of this oil was the reason for choosing it). The formulated-with-antioxidant and control samples were studiedat 25 ° C and 180° C during 45 days of storage onthe days 0, 15,30 and 45.
Oil stability
Measurement of the Peroxide value
Primary oxidation products, hydroperoxides, were determined by the peroxide value measurements. 1_0.1 g of the oil was weighed and subjected to the iodometric determination of the peroxide value (AOCS 1999).
The induction period was considered as the number of days needed for the peroxide value of the sample to reach 20 meq O2/kg of the edible oil (Economou et al 1991).
Measurement of the Iodine value
The oil samples, weighing 0.25 g,were poured into a completely dry 500-ml flask. Then, each sample was dissolved in 10 ml of chloroform and 25 ml of the Hanus reagent was added to the solution with a bubble pipette. The Erlenmeyer flasks were capped, kept in the dark, and shaken every once in a while. After 30 minutes, 20 ml of 15 % potassium iodide solution was added to each Erlenmeyer flask and the flasks were vigorously shaken. 100 ml of freshly boiled and cooled distilled water were poured into each flask, taking care that the cap and the inside walls of the flasks were also washed so that any excess iodide, adhering to them, was also washed into the solutions inside the flasks. The solutions were then titrated by using 0.1 N sodium thiosulfate. The contents of each flask were repeatedly stirred and the titration went on until the solution turned yellow. At this stage, 2 – 3 drops of the starch reagent were added and the titration continued till the blue color completely disappeared. Care was taken to vigorously shake the flasks near the end of the titration so that any iodine particles remaining in chloroform were absorbed by the potassium iodide solution. Together with the oil samples, the test was also performed on the control. The iodine value was calculated using equation 1:
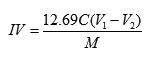
where N is the normality of the sodium thiosulfate solution, B denotes the ml of the thiosulfate used for the control sample , S represents theml of the thiosulfate used for the samples, W stands for the weight of the sample (g), and 126.9 is the molecular weight of iodine (AOAC 2005).
Measurement of the Anisidine value
p-Anisidine was dissolved in glacial acetic acid to make up a 0.25g/100ml solution. Isooctanewas used as a solvent for the oil samples. The test was conducted in triplicate for all samples. 0.5-0.7g of the oil sampleswas accurately weighed in 25-ml volumetric flasks, noting down the masses of the samples used. They were then diluted to the volume with isooctane. The absorbance values (Ab) of the resulting solutions were measured at 350 nm using isooctane as blank. Glass cuvettes were used for all absorbance measurements. 5ml of the solution was then transferred to a test tube and 5ml of isooctane to another test tube. 1ml of the p-anisidine solution was added to both of them and the solutions were mixed. After 10 minutes, the absorbance values (Ab) of the sample solutions were read with isooctane as blank. The p-Anisidine values were calculated using the following equation (Eq.2): (AOAC 2005).
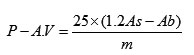
Measurement of the Acid value
Each oil sample (1.0 g) was weighed and dissolved in 50 ml of ethanol in a conical flask. Two drops of phenolphthalein were added and titrated to pink end point (which persisted for 15 minutes) with 0.1 N potassium hydroxide (KOH). Acid value was calculated as follows:
Acid value = 56.1 V C m
where 56.1 is the equivalent weight of KOH, V is the volume (ml) of the standard volumetric KOH solution used, C is the exact concentration of the KOH solution used (0.1 N); m is the mass (g) of the test portion (1 g).(AOAC 2005).
Evaluation of the antioxidant activity by the Rancimat method
The method used, was adapted from Gortziet al. (2006). The antioxidants namely, BHT, TBHQand APEWN all at 150 ppm -were accurately added to stripped sunflower oil (Elais S.A., Athens, Greece) and the samples prepared using the method described by Fuster et al., (1998) and their effects were determined using a Rancimat 679 (Metrohm LTD, Herisau, CH 9101, Switzerland), along with another sample of sunflower oil without antioxidant (control). 1 mL of the appropriate solvent (MeOH or CH2Cl2) was added in order to dissolve the antioxidant. The conditions were set at 90 °C and 15 L/h.
The protection factor (P.F.) was calculated as:
P.F. = (induction period with antioxidant)/(induction period without antioxidant).
A protection factor greater than 1 indicates the inhibition of the lipid oxidation. The higher value represents the better antioxidant activity.
Statistical analysis
The data analysis of variance (ANOVA) was performed using SPSS version 17. Duncan`s multiple rangetest was carried out for mean comparison at the 95% significance level (p<0.05). Each treatment was repeated in triplicate.
Results and Discussion
Peroxide values (PV)
PV is widely used as a measure of the primary lipid oxidation, indicating the amount of peroxides formed in fats and oils during oxidation (Ozkan et al. 2007). The effects of BHA, BHT and APEWNadded to the sunflower oil samples, are shown in fig 1.It was regular increase in PV for all the samples over the storage period.This rise can be attributed to the formation of hydro-peroxides as the initial products of the oxidation of the oil samples. The total increment in PV is as follows in descending order: SFO (control) >APEWN >BHT>TBHQ (Fig.1). There were no significant differences between the peroxide values of the oil samples formulated withAPEWN and BHT during 15 days of storage, but TBHQ had a lower peroxide value during this period. However, when the sunflower oil samples were stored for 30 to 45 days, APEWN was more effective in controlling the peroxide value, thus the oxidation of the sunflower oil samples was delayed and the antioxidant activity was enhanced with APEWN during storage.
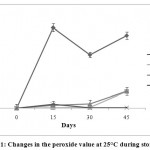 |
Figure 1: Changes in the peroxide value at 25°C during storage. |
The results of the peroxide value measurements at 180°C showed that the total PV was increased as follows:SFO control >APEWN ≥ BHT ≥ TBHQ (Fig.2). There were no significant differences between the peroxide values of the oil samples containing BHT and TBHQ and APEWN at 180°Cduring storage. The addition of APEWNbrought about a more intense antioxidant activity (significant at P<0.05). This means thatAPEWN, as a natural antioxidant, can substitute for synthetic antioxidants in reducing the peroxide values of the sunfloweroil samples.
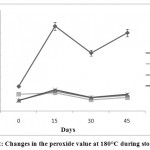 |
Figure 2: Changes in the peroxide value at 180°C during storage. |
In that phase, the formation of hydroperoxides wasshown by their decomposition into secondaryproducts.Yanishlieva et al.(1996) declared thatascorbylpalmitatewas a better antioxidant in oils.Liolios et al.(2009) showedthat the carvacrol encapsulated with nanoliposome presented a better antioxidant effect compared with the pure samples.Satyanarayana et al.(2000)stated that ascorbylpalmitate helped control the formation of hydroperoxides in fryingoils in comparison with other antioxidants, including BHA, BHT, PG. They also cited that the addition of ascorbylpalmitateto frying oil reduced the PV of the extracted fat during storage.Vayupharp and Laksanalamai (2012) claimed that the incorporation ofgrape seed into fried pork inhibited or delayed rancidity, and also there was no statistically significant difference in the rancidity as shown by the PV values of the fried pork with BHT and grape seeds.
Iodine value
The iodine value is a measure of the unsaturation of oils. It is one of the parameters used to measure the oil quality (Rehab 2012). Fig 3 demonstrates the IV of the sunflower oilformulated with various types of antioxidants at 25 °C. Sunflower oil with various levels of anantioxidant during storage at 25 °C experienced a significant (P<0.05) decrease in iodine values (degree of oil unsaturation).This decrease was due to the increase in the predominance of monounsaturated fatty acids in the oil samples. The initial iodine values of the control were 131.99to 60 g, but 132.01 to 92 g in sunflower oil with antioxidant.The antioxidant reduced the oxidation rate of PUFAs, as detected by the relatively low reduction in the iodine values. There were no significant differences between the iodine valuesof the oil samples formulated withAPEWN and BHT and TBHQ during storage at 25 °C (Fig.3).
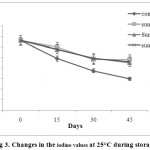 |
Figure 3: Changes in the iodine values at 25°C during storage. |
The IV of sunflower oil with different antioxidants during deep fat frying at 180°C ± 5°Care depicted in Fig.4. The frying process induced a significant (P<0.05) decrease in the IV of all oil samples under study. It is well known that during frying, some of the non-conjugated double bonds are converted to conjugated ones. The conjugated system, in general, precludes the complete addition of iodine (Rehab 2012). This fact indicates the decrease of IV for the samples under study during frying at 180 °C ± 5 °C for 45 daysof storage.The decrease in iodine value denotes the decrease in the degree of unsaturation of the oil caused by the extent of oxidation (Kirk andSawyer 1991; Rehab 2012). The highest decrease in IV was recorded for the sunflower oil without antioxidant due tothe reduction percentage in the iodine valuewhichranged from 118.03 to 75 at the end of the frying period. On the other hand, the samples withAPEWN, BHT and TBHQhad significantly the smallest reduction in their iodine values which were 95, 92.06 and 96 at the end of the frying period, respectively. Autoxidation of the samples affected their fatty acid composition, as polyunsaturated fatty acids were oxidized faster than the saturated and mono-unsaturated ones(Semwal et al. 1996). The addition of antioxidant to sunflower oil during frying effectively reduced the oxidation rate in sunflower oil, as detected by the relatively low reduction in the iodine values (fig 4). The results showed that APEWN was not significantly different from BHT and TBHQ which can replace them as a natural antioxidant.
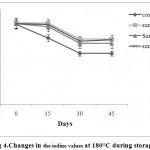 |
Figure 4: Changes in the iodine values at 180°C during storage. |
Anisidine value
During lipid oxidation, hydroperoxides, the primary reaction products, decompose to produce secondary oxidation products (aliphatic aldehydes, ketones, alcohols, acids, and hydrocarbons) which are more stable during the heating process, responsible for off-flavors and off-odors of edible oils (Poiana 2012). Sunflower oil had the highest p-anisidine values,because of oleic acid and linoleic acid, the main types of unsaturated fatty acids that were oxidized, present in the highest amount ofsunflower oil (88%)(Allen and Hamilton 1989).
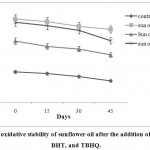 |
Figure 5: The oxidative stability of sunflower oil after the addition of APEWN, BHT, and TBHQ. |
The control sample (without anyextract) had the highest p-anisidinevalue and thus indicated a higher rate ofoxidation. Among different methanolic and acetone extracts, 80% methanolic(Table 1) presented the changes in p-AV at25°C and 180°C as affected by supplementation with APEWN, BHT, and TBHQ. The control sample (without any antioxidant) had the highest p-anisidinevalue and thus indicated a higher rate of oxidation.
Table 1: Changes inp-anisidine values of sunflower oil with antioxidant
| sample | 0 day | 15 day | 30 day | 45 day | ||||
| 25°C | 180°C | 25°C | 180°C | 25°C | 180°C | 25°C | 180°C | |
| control | 0.71±0.16a | 6.95±1.12a | 36±2.09a | 38±2.31a | 51±2.36a | 53±2.34a | 60±2.4a | 65±2.5a |
| TBHQ | 0.717±0.25a | 4.86±1.14d | 22±2.11d | 24±2.45d | 30±2.61d | 29±2.22c | 41±2.4d | 45±2.69d |
| BHT | 0.72±0.17a | 5.42±1.23b | 33±2.16b | 35±2.33b | 39±2.64b | 34±2.29b | 51±2.52b | 52±2.3b |
| APEWN | 0.7±0.22a | 5.03±1.15c | 26±2.08c | 28±2.51c | 35±2.66c | 30±2.18c | 47±2.48c | 47±2.54c |
The addition of APEWN, BHT, and TBHQ resulted in a significant decrease in p-AV (p < 0.05. The result of p-AV at 25°C and 180°C showed that the total p-AV was increased as follows: SFO control > BHT ≥ APEWN ≥ TBHQ. TBHQ provided the best protection against the secondary oxidation of the oil samples. However, SFO treatments withAPEWN showed reducedp-AVs compared with those ofBHT.
Poiana(2012)reported that grape seed extract (GSE) provided the best protection against the secondary oxidation of the sunflower oil samples in comparison with BHT. They attributed these results tothe polyphenolic compounds found in GSE that had a strong inhibitory effect on the secondary lipid oxidation.
Acid value
The FFA contents of SFO (control), SFO-TBHQ, SFO – BHT and SFO – APEWN during 45 days of accelerated storageat 25°C and 180°C are shown in Table 2. When comparing among different samples, the total increment of the FFA content during 45 days of storage was in the order of SFO (Control) > SFO – BHT > and SFO – APEWN > SFO-TBHQ. APEWN exhibited excellent antioxidant activity in comparisonwith BHT. The FFA of SFO from day 0 till day 45 and at the frying temperature with APEWN controlled the FFA oxidation well throughout the storage period.
Table 2: Changes in the acid values (mg KOH/g Oil) of the sunflower oil samples with antioxidant
| sample | 0 day | 15 day | 30 day | 45 day | ||||
| 25°C | 180°C | 25°C | 180°C | 25°C | 180°C | 25°C | 180°C | |
| control | 0.05±0.0 | 0.05±0.0 | 2.51±0.03 | 2.08±0.03 | 4±0.2 | 4.05±0.34 | 3.2±0.4 | 3.05±0.5 |
| TBHQ | 0.05±0.0 | 0.05±0.0 | 1.04± .04 | 1.06±0.02 | 1.08±0.04 | 2±0.22 | 1.59±4 | 1.09±0.09 |
| BHT | 0.05±0.0 | 0.05±0.0 | 1.06±0.03 | 1.09±0.08 | 2±0.4 | 2.04±2 | 1.89±52 | 2±0.3 |
| APEWN | 0.05±0.0 | 0.05±0.0 | 1.06±0.03 | 1.07±0.06 | 1.5±0.3 | 2±0.18 | 1.67±4 | 1.4±0.2 |
The antioxidant activity
The fat and oil oxidation stability is commonly assessed by the fully automated version of active oxygen method available in Rancimat apparatus and is accepted as a standard method by American Oil Chemists’ Society (AOCS (Cd 12b-92) 1999) (Hamed et al. 2012). Rancimat method determines the induction period by measuring the increase in volatile acidic by-products released from the oxidizing fat at 100- 110 °C. The concentration of degradation products which are transferred to distilled water is monitored by measuring conductivity. Longer induction periods suggest thestronger activity of the added antioxidants(Hamed et al. 2012).
The control sample (sunflower oil without any antioxidant) showed the shortest induction period (3.28 hr). The descending order of antioxidant capacity was TBHQ>APEWN >SFO – BHT >control (Fig.5).Hamed et al. (2012) suggested that TBHQ revealed the highestprotection as indicated by its longest induction period (12.3 hr).
Špiclinet al. (2001) expressedthe stability of ascorbylpalmitate is highly dependent on its initial concentration, its location in the microemulsion, the amount of oxygen dissolved in the system and the storage conditions.Possible approaches to increase its stability include the proper selection of the type of microemulsion and the appropriate choice of initial concentration and storage conditions. Other factors also have to be considered in formulating an optimal microemulsion as a carrier for ascorbylpalmitate, for example, the selection ofnon-oxidizable components of the carrier system. Nevertheless,ascorbylpalmitate is still convenientas an antioxidant to stabilize formulations.
The modified antioxidant action of the extract resulting from its encapsulation was expected, sincethe complex (liposome membrane-fraction) possesses new physicochemical characteristics and bioactivity dependant on structure, size and ξ-potential of the preparation. Liposomes containing the extracts were more stable than those extract-free. This may imply that lipophilic substances used in the extracts and phospholipids were packed regularly and tightly, conferring the membrane rigidity and decreasing the membrane permeability (Gortzi, et al.2007).Jurkovic et al.(2003) appliedascorbylpalmitate in microemulsions for scavenging free radicals formed in UV irradiated porcine skin. ascorbylpalmitateacts as an antioxidant. Due to its chemical structure and amphiphilicnature, the molecules of ascorbylpalmitate are orientated in the lipid bilayers with the palmitic residue in the lipophilic phase and the lactone ring in the lipid–water interphase. They suggested the effectiveness of ascorbylpalmitate was dependent on its concentration and the type of microemulsion.HrasÏ et al. (2000) showed the higherantioxidant activity of ascorbylpalmitate in sunflower oil compared with α- tocopherol and citric acid.
Conclusions
The present study has demonstrated the potential antioxidant preservativability of ascorbylpalmitate. Encapsulation in liposomes modified the activities of ascorbylpalmitate.Ascorbylpalmitatewas compared with BHT and TBHQ, BHT and TBHQ are synthetic antioxidant products, widely used in oil. Our results suggested that APEWN would be an effective natural antioxidant in sunflower oil. These results also suggested that ascorbylpalmitate has the potential to be used as a naturally derived antioxidant for specialized food application in processed food products.
References
- American Oil Chemists’ Society (AOCS), Official Methods and Recommended Practices of the American Oil Chemists’ Society. 1999. Campaign: American Oil Chemists’ Society.
- Association of Official Analytical Chemists.Official Methods of Analysis. 2005.19thEdition, Washington: AOAC International.
- Allen J.C. and Hamilton R.J. “Rancidity in Foods”. 1989. 2nd ed. Pub. Elsevier Science, England.
- Colas, J. C., Shi, W., Rao, V. S. N. M., Omri, A., Mozafari, M. R., & Singh, H. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron, 2007.38(8), 841–847.
- CORT, W.M. Antioxidant activity of tocopherolsascorbylpalmitate and ascorbic acid and their mode of action. 1974. J. Am. Oil Chem. SOC. 5 I ,32 1-325.
- EbrahimiKhoosfi, M., Khosravi-Darani, K.,Hosseini, H., Arabi, Sh., Komeili, R., KoohiKamali, P. Production of ZatariamultifloraBoiss Essential Oil Nanoliposomes by Response Surface Methodology, Nanoscale, 2014. 1(1): 15-18.
- Economou, K. D., Oreopoulou, V., &Thomopoulos, C. D. Antioxidant activity of some plant extracts of the family Labiatae. Journal of American Oil Chemists’ Society, 1991.68, 109-113.
- Fuster, M. D.; Lampi, A. M.; Hopia, A.; Kamal-Eldin, A. Effects of α- and γ-tocopherols on the autoxidation of purified sunflower triacylglycerols. Lipids1998, 33, 715-722.
CrossRef - Gregoriadis, G. Liposome technology.Liposome preparation and related techniques (3rd ed., Vol.I). 2007. New York: Informa Healthcare Inc.
- Gortzi, O.; Lalas, S.; Chinou, I.; Tsaknis, J. Evaluation of the Antimicrobial and Antioxidant Activities of OriganumdictamnusExtracts before and after Encapsulation in Liposomes. Molecules 2007, 12, 932-945
CrossRef - Gortzi, O.; Lalas, S.; Chinou, I.; Tsaknis, J. Reevaluation of antimicrobial and antioxidant activity of Thymus spp. extracts before and after encapsulation in liposomes. J. Food Protect. 2006, 69, 2998-3005.
CrossRef - Hamed,S.F., Wagdy, S. M., and Megahed, M.G. Chemical Characteristics and Antioxidant Capacity of Egyptian and Chinese Sunflower Seeds: A Case Study. Life Science Journal; 2012.9(2)
- HrasÏ, A. R., Hadolin, M., Knez, Z., Bauman, D. Comparison of antioxidative and synergistic effects of rosemary extract with a-tocopherol, ascorbylpalmitate and citric acid in sunfower oil. Food Chemistry, 2000. 71: 229-233.
CrossRef - Huber, G.M., VasanthaRupasinghe, H.P. and Shahidi, F. Inhibition of oxidation of omega-3 polyunsaturated fatty acids and fish oil by quercetin glycosides. Food Chemistry, 2009.117 (2): 290-295.
- Khanniri E., Bagheripoor-Fallah N., Sohrabvandi S., Mortazavian, A. M. Khosravi-Darani, K., Mohammad R. Application of Liposomes in Some Dairy Products, Critical Reviews in Food Science and Nutrition , 2016,0:1–10.
- Khan, M. A., &Shahidi, F. Tocopherols and phospholipids enhance the oxidative stability of borage and evening primrose triacylglycerols. Journal of Food Lipids, 2000, 7(3), 143–150.
CrossRef - Khosravi-Darani K. and Mozafari M.R., Nanoliposome Potentials in Nanotherapy: a Concise Overview, International Journal of Nanotechnology and Nanoscience, Vol 2010, 6: 1: 3-13.
- Khosravi-Darani, K., Mozafari, M. R. Rashidi, L. Calcium Based Nonviral Gene Delivery: An Overview of Methodology and Applications, Acta MedicaIranica, ActaMedicaIranica, 2010, Vol. 48, No. 3, pp: 133-141.
- Khosravi-Darani, K., Pardakhty, A., Honarpisheh, H., Rao, V.S.N.M., Mozafari, M.R.The role of high-resolution imaging in the evaluation of nanosystems for bioactive encapsulation and targeted nanotherapy, 2007, Micron
CrossRef - KhosraviDarani, K., EbrahimiKhoosfi, M., Hosseini, H., Encapsulation ofZatariaMultifloraBoiss.Essential oil in liposome: antibacterial activity against E. coli O157:H7 in broth media and minced beef, Journal of Food Safety, 2016 In Press, doi: 10.1111/Jfs.12271.
CrossRef - Kirk RS, Sawyer R. Pearson’s Composition and Analysis of Foods.(9thedn). Longman Scientific and Technical England, 1991, 607-617.
- Krishnaiah, D., Sarbatly, R. and Nithyanandam, R. A review of the antioxidant potential of medicinal plant species.Food and Bioproducts Processing, 2010, 157: 1-17.
- Kristl, J., Volk, B., MirjanaGasˇperlin, M., Sˇentjur, M., Jurkovic. P. E ffect of colloidal carriers on ascorbylpalmitate stability. European Journal of Pharmaceutical Sciences, 2003, 19: 181–189
https://doi.org/10.1016/S0928-0987(03)00104-0z
CrossRef - Liolios, C.C. Gortzi, O. Lalas, S., Tsaknis, J. Chinou I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanumdictamnus L. and in vitro antimicrobial activity. Food Chemistry, 2009, 112; 77–83
CrossRef - Jahadi, M., Khosravi-Darani, K., Ehsani, M.R., Mozafari, M.R., Saboury, A.A., Pourhosseini, P.S.
- The encapsulation of flavourzyme in nanoliposome by heating method, Journal of Food Science and Technology, Volume 52, Issue 4, 1 April 2015, Pages 2063-2072
- Jahadi, M., Khosravi-Darani, K., Ehsani, M.R., Seydahmadian, F., Vafabakhsh, Z.Evaluating the effects of process variables on protease-loaded nano-liposome production by Plackett-Burman design for utilizing in cheese ripening acceleration, Asian Journal of Chemistry, 2012, 24, No. 9: 3891-3894.
- Jurkovic, P., Sentjurc, M., Gasperlina, M., Kristla, J., Skin S. P. Protection against ultraviolet induced free radicals with ascorbylpalmitate in microemulsions. European Journal of Pharmaceutics and Biopharmaceutics, 2003, 56; 59–66.
CrossRef - Vayupharp B, Laksanalamai V. Recovery of Antioxidants from Grape Seeds and its Application in Fried Food. J Food Process Technol, 2012, 3:152.
CrossRef - Maherani, B., Arab-Tehrany, E., Kheirolomoom, A., Cleymand, F., Linder,M. Influence of lipid composition on physicochemical properties of nanoliposomes encapsulating natural dipeptide antioxidant L-carnosine. Food Chemistry 2012, 134: 632–640.
CrossRef - McMullen, L.M., Hawrysh, Z.J., Lin, C. and Tokarska, B. Ascorbylpalmitate efficacy in enhancing the accelerated storage stability of canola oil. J. Food Sci. 1991, 56:1651.
CrossRef - Min, D.B., Lee, H.O. Lipid oxidation of edible oil, Food lipid. In: Akoh, C.C. & Min, D.B. (Eds.): Chemistry, Nutrition, and Biochemistry, 1998, 88, 283-96.
- Mozafari, M. R., Khosravi-Darani, K., Borazan, G. G., Cui, J., Pardakhty, A., &Yurdugul, S. Encapsulation of food ingredients using nanoliposome technology. International Journal of Food Properties, 2008, 11(4), 833–844.
CrossRef - Mozafari, M.R., Khosravi-Darani, K.,An overview of liposome-derived nanocarrier technologies. In: Nanomaterials and Nanosystems for Biomedical Applications (Ed. Mozafari, M.R.) 2007, Springer
CrossRef - Mortazavi, S.M., Mohammadabadi, M.R., Khosravi-Darani, K., Mozafari, M.R.Preparation of liposomal gene therapy vectors by a scalable method without using volatile solvents or detergents , Journal of Biotechnology, 2007,129: 604-613.
CrossRef - Ozkan, G, Simsek B, Kuleasan H. Antioxidant activity of Saturejacilicica essential oil in butter and in vitro. J. Food Eng, 2007. 79:1391-1396.
CrossRef - Poiana, M. A. Enhancing Oxidative Stability of Sunflower Oil during Convective and Microwave Heating Using Grape Seed Extract. Int. J. Mol. Sci. 2012, 13, 9240-9259.
CrossRef - Rasti, B., S. Jinap, S., M.R. Mozafari, M. R., A.M. Yazid, A. M. Comparative study of the oxidative and physical stability of liposomal and nanoliposomal polyunsaturated fatty acids prepared with conventional and Mozafari methods. Food Chemistry, 2012, 135: 2761–2770
CrossRef - Rehab, F.M.A. Improvement the stability of fried sunflower oil by using different levels of Pomposia (SyzyygiumCumini).Electron. J. Environ. Agric. Food Chem. 2010, 9, 396–403.
- R eynolds, J.E.F. (Ed.), Martindale, The Extra Pharmacopoeia, 31st Edition. Royal Pharmaceutical Society, London.1996.
- Satyanarayana, A., Giridhar, N., Josh G. J., and Rao’.D.G.Ascorbylpalmitate as an antioxidant for deep fat frying of potato chips in peanut oil. Journal of Food Lipids, 2000, 7:1-10.
CrossRef - Semwal AD, NarasimhaMurtby MC, Sharma GK, Arya SS. Studies on storage stability of commercially marketed refined sunflower oil in plastic film packs. J Food SciTechnol, 1996, 33: 352-354.
- Siddhuraju, P., Beeker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro climatic origins of Drumstick tree (Moringaoleifera Lam.) leaves. Journal of Agriculture and Food Chemistry, 2003, 51: 2144-55.
CrossRef - Shahidi, F. and Wanasundara, U.N. Methods for evaluation of the oxidative stability of lipid-containing foods. Food Science and Technology International, 1996, 2 (2): 73-81.
- S ˇ piclin, P.Gasˇperlin, M., Kmetec, V. Stability of ascorbylpalmitate in topical microemulsions, Int. J. Pharm.2001, 222:271–279.
CrossRef - Vafabakhsh, Z., Khosravi-Darani K,Khajeh, K., SeyedMortazavian, A. M., Jahadi, M., Komeili, R. Stability and catalytic kinetics of protease loaded liposomes, Biochemical Engineering Journal, 2013, (72) 11-17.
CrossRef - Yanishlieva, N. V., &Marinova, E. M. Antioxidative effectiveness of some natural antioxidants in sunflower oil.ZeitschriftfuÈrLebensmittel-Untersuchung und Forschung, 1996, 203:220±223.

This work is licensed under a Creative Commons Attribution 4.0 International License.





