How to Cite | Publication History | PlumX Article Matrix
Cloning and in Vitro Expression of the Human Alpha‑Fetoprotein Gene
Gulshan Stanbekova1,2, Leila Nadirova1,2, Daniyar Beisenov1,2, Nailya Polimbetova1,2 and Bulat Iskakov1,2
1Aitkhozhin Institute of Molecular Biology and Biochemistry, 86, Dosmukhamedov Str., Almaty, Kazakhstan.
2Institute of Plant Biology and Biotechnology, 45, Timiryazev Str., Almaty, Kazakhstan.
Corresponding Author E-mail: beisenov.d@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2361
ABSTRACT: Alpha-fetoprotein is known as a tumor marker. Strong conjugating features of the alpha-fetoprotein and its ability for specific binding with receptors of the tumor cells offers opportunity for the targeted delivery of cytostatic and cytotoxic drugs into malignant cells. Human AFP are able to induce apoptosis and to inhibit growth of the tumor cells. Technology of the recombinant DNA is the way to simplify and cheapen the production of the human AFP. Complementary DNA encoding human alpha-fetoprotein was cloned into bacterial vectors under the control of the T7 bacteriophage promoter. Various 5'- and 3'- untranslated regions (5'- and 3'-UTRs) were used as a translational enhancers. Transcription of the recombinant constructs by the T7 RNA polymerase and subsequent in vitro translation of the resulted transcripts in the wheat germ cell-free system were performed. It was shown that the human fetal blood protein could be correctly synthesized in plant cell-free system.
KEYWORDS: AFP; translational enhancers; in vitro translation; wheat germ cell-free system
Download this article as:| Copy the following to cite this article: Stanbekova G, Nadirova L, Beisenov D, Polimbetova N, Iskakov B. Cloning and in Vitro Expression of the Human Alpha‑Fetoprotein Gene. Biosci Biotech Res Asia 2016;13(4). |
| Copy the following to cite this URL: Stanbekova G, Nadirova L, Beisenov D, Polimbetova N, Iskakov B. Cloning and in Vitro Expression of the Human Alpha‑Fetoprotein Gene. Biosci Biotech Res Asia 2016;13(4). Available from: https://www.biotech-asia.org/?p=17152 |
Introduction
Alpha-fetoprotein (AFP) is a mammalian embryo-specific protein, also known as a tumor marker and could be found in some cases of liver and reproductive system cancers1.
Human AFP (AFPh) is a 70 kDa glycoprotein containing up to 4 % of carbohydrates. It is a transport protein possessing strong conjugating features. It is known that some receptors of tumor cells are AFP ligands2. This feature is used for the targeted delivery of cytostatic and cytotoxic drugs into malignant cells3,4,5,6,7. Development of the drugs inducing apoptosis in the tumor cells is one of the perspective directions in oncology. The ability of the AFPh to induce apoptosis and to inhibit growth of some tumors by expression of the p53 gene were shown in several researches8,9,10,11,12,13,14.
Alternatively, AFP conjugation with antisense oligonucleotides to the mRNAs of the genes participating in cell proliferation and apoptosis could make sense. AFP conjugate with an antisense oligonucleotide for the mRNA of the antiapoptotic bcl-2 gene induced apoptosis and subsequent death of the tumor cells15.
Thus, biological features of the AFPh offers great opportunities to develop various anticancer drugs that could be highly selective to the tumor cells. However, this promising perspective is limited to the complexity and the cost of the AFPh purification from the abortion serum. Recombinant systems producing AFPh could be the solution.
The aims of this work were AFPh gene cloning and optimization of its expression in a plant in vitro system.
Materials and Methods
Human hepatocellular carcinoma (HepG2) cell line was used as a DNA source.
RNA was purified by commercial “RNAqueous®” kit using manufacturer’s guide (Ambion).
The revers transcription reaction was driven by the use of the moloney murine leukemia virus reverse transcriptase (M-MLV RT) (Thermo Scientific). The subsequent polymerase chain reaction was directed by the PL polymerase (Syntol).
DNA cloning was performed according to 16.
DNA sequencing by Sanger was made in “ABI PRISM 3101 Genetic Analyzer” (Applied Biosystems) using “BigDye® Terminator v3.1 Cycle Sequencing Kit” according to manufacturer’s recommendations (Applied Biosystems).
Sequence of the 5′-UTRs with underlined HindIII and NcoI restriction sites.
[pl]: AAGCTTAGTCGACCATGG
[2xARC]: AAGCTTACAAATACTCCCCCACAACAGCTTACAAATACTCCCCCACAACAGCTTGTCGACCATGG
[3xARC]: AAGCTTACAAATACTCCCCCACAACAGCTTACAAATACTCCCCCACACAGCTTACAAATACTCCCCCACAACAGCTTGTCGACCATGG
[PVY]: AAGCTTAATTAAAACAACTCAATACAACATAAGAAAAACAACGCAAAAACA
CTCATAAACGCTCATTCTCACTCAAGCAACTTGCTAAGTTTCAGTTTAAATCATTTCCTTGCAATTCTCTAGAACAATATTGGAAAC CATTTCAAC TCAACAAGCAATTTCATCACTTCCAACCAATTTCAGATCCTCСATGG
In vitro transcription
In vitro transcription by the T7 RNA-polymerase according to 17. 50 µL of the reaction mix contained 40 mM Tris-HCl (pH 7.9), 6 mM MgCl2, 10 mM DTT, 10 mM NaCl, 2 mM spermidin, 2 mM of each ribonucleoside triphosphate, 40 units of the ribonuclease inhibitor, 40 units of the T7 RNA-polymerase and 1 µg of DNA. The reaction lasted 2 hours at 37°C. Then the reaction mix was treated with chloroform. RNA precipitated in the presence of 3M LiCl. The pellet was washed with 70% ethanol after centrifugation at 14000 RPM. Dried under vacuum the pellet was dissolved in H2O. RNA concentration was determined by spectrophotometry.
In vitro translation
In vitro translation was carried out in the wheat germ cell-free system from the “Kazakhstanskaya 4” variety18. 25 µL of the reaction mix contained 20 mM Tris-Ac (pH 7.6), 90 mM KAc, 2 mM MgAc2, 10 mM phosphocreatine, 0.12 mg/mL creatine phosphokinase, 1 mM ATP, 0.1 mM GTP, 0.1 mM spermidin, 0.1 mM of each aminoacid except methionine, 0.025 mM methionine, 0.075 mM [35S]-methionine, 1 µg of mRNA, 7 µL of the wheat germ extract. The reaction mix was incubated for 1 hour at 26°C.
Autoradiography and western blot analysis
Protein electrophoresis was performed according to the classical Laemmli protocol19. The gel was dried and subsequent autoradiography using “Kodak” X-ray film was made. Western blot analysis was made according to 20. Antibodies conjugated to horseradish peroxidase were used as a secondary to AFPh-specific monoclonal antibodies.
Results and Disscussion
AFP gene belongs to the albumin gene family and located in the long arm of the fourth chromosome (4q11-q13)21. Like all secreted proteins, AFP is synthesized as a precursor, which becomes the mature protein after cleavage of the signal peptide and the process of glycosylation. The complete nucleotide sequence of the AFP gene is known. It consists of 20000 base pairs, contains 15 exons and 14 introns22.
Nucleotide sequence of the AFP mRNA consists of 2098 nucleotides and has three main parts: 44 noncoding nucleotides at the 5′-end, 1830 coding and 155 noncoding nucleotides at the 3′-end23. The signal peptide consists of 19 amino acids followed by 590 amino acids of the mature protein.
Thus, taking into account big size of the AFPh gene and many introns inside it seems impossible to express it neither in vitro nor in vivo in bacterial or plant systems. We decided to clone complementary DNA (cDNA) of the AFPh gene. mRNA sequence of the AFPh (GenBank: NM_001134) was analyzed with SnapGene® software (GSL Biotech) to build DNA restriction map. These data were used to design oligonucleotide primers. Total RNA was purified from the AFPh producing human hepatocellular carcinoma (HepG2) cell line (Fig. 1). It is seen from the Fig. 1 that 28S, 18S ribosomal RNAs and 4S transport RNA (about 100 nucleotides) are major components of the sample. The RNA sample was enriched by highly weight molecular components after precipitation by 3M LiCl, since low weight tRNA molecules was leaved in the supernatant fraction (lane 2).
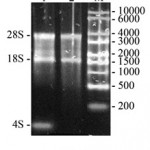 |
Figure 1: Nucleic acids purified from the human hepatocellular carcinoma (HepG2) cell line and separated by electrophoresis in 1% agarose gel. 1 – total nucleic acid sample; 2 – nucleic acid sample after precipitation by 3M LiCl; M – RNA marker (size marked in nucleotides on the right). Positions of the 28S rRNA, 18S RNA and 4S tRNA are marked on the left. |
Obtained RNA sample was used to synthesize cDNA of the AFPh by moloney murine leukemia virus reverse transcriptase with the use of antisense primer no.1 (5’GCATCTCGAGTTAAACTCCCAAAGCAGCACGAGTTTTTG) containing XhoI restriction site (underlined). Antisense oligonucleotide primer no.1 and sense oligonucleotide primer no.2 (5’GCAACCATGGAGTGGGTGGAATC), containing NcoI restriction site (underlined) were used to amplify the resulting cDNA. We performed 30 cycles of the following PCR program: 30 seconds at 94°C (initial denaturing lasted 4 minutes), 30 seconds at 48°C and 1 minute at 72°C (final elongation lasted 5 minutes). Results of the RT-PCR are represented in the Fig. 2.
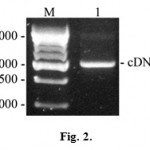 |
Figure 2: RT-PCR products separated by electrophoresis in 1% agarose gel. M – DNA marker (size marked in nucleotide pairs on the left); 1 – cDNA of the AFPh. |
Electrophoregram shows resulting 2000 bp DNA amplification products presumably coding AFPh protein.
2000 bp fragments obtained were eluted from the gel and cloned into series of bacterial pIVEX-WG-1.3 vectors between NcoI-XhoI restriction sites under the control of the T7 bacteriophage promoter. The plasmids of this series differ from the analogous vectors constructs by the presence of the translational enhancers described earlier24. In addition, it is possible to insert various 5′-untranslating regions between HindIII-NcoI restriction sites to test if it increases efficiency of the mRNA translation.
The resulting recombinant plasmids, containing AFPh gene are schematically represented in the Fig. 3.
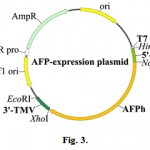 |
Figure 3: Schematic representation of the plasmids for in vitro expression of the AFPh gene. Vectors with the molecular size about 5000 bp contain promoter of the bacteriophage T7, one of the 5′-UTR, AFPh gene and the 3′-UTR of the tobacco mosaic virus (3′-TMV). |
We performed DNA sequencing of the cloned AFPh gene from the 3′-end (about 500 nucleotides). Comparative Blast-analysis identified the sequence as AFPh mRNA from the NCBI “Nucleotide” database (www.ncbi.nlm.nih.gov).
Thus, we developed three types of the recombinant plasmids that could be transcribed by the T7 RNA-polymerase and differing only in the structure of the 5′-UTR.
In subsequent experiments, the plasmids were linearized with EcoRI enzyme, which unique restriction site is situated after the 3′-UTR of the TMV genomic RNA. This linearized plasmids served as a template for in vitro transcription from T7 promoter. Equal amounts of uncapped mRNAs synthesized by the T7 RNA-polymerase were used as a templates for in vitro translation in the wheat germ cell-free system.
Proteins translated in vitro in the presence of the [35S]-methionine were separated in 12% sodium dodecyl sulfate polyacrylamide gel (SDS-PAG). Then PAG was dried and exposed with X-ray film. Results are represented in the Fig. 4.
![Figure 4: Autoradiography of the translation products from various mRNAs in the wheat germ cell-free system in the presence of the [35S]-methionine. M – protein marker (size marked in kDa on the left); 1 – AFPh translation products from the mRNA with the control 5'-UTR [pl]; 2 – AFPh translation products from the mRNA with the 5'-UTR [3xARC]; 3 – GFP translation products from the mRNA with the 5'-UTR [PVY]; 4 – GFP translation products from the mRNA with the 5'-UTR [2xARC].](https://www.biotech-asia.org/wp-content/uploads/2016/12/Vol_13_no4_Clon_Guls_fig4-150x150.jpg) |
Figure 4: Autoradiography of the translation products from various mRNAs in the wheat germ cell-free system in the presence of the [35S]-methionine. M – protein marker (size marked in kDa on the left); 1 – AFPh translation products from the mRNA with the control 5′-UTR [pl]; 2 – AFPh translation products from the mRNA with the 5′-UTR [3xARC]; 3 – GFP translation products from the mRNA with the 5′-UTR [PVY]; 4 – GFP translation products from the mRNA with the 5′-UTR [2xARC]. |
As you can see from the Fig. 4, the presence of the 5′-UTRs [PVY] (lane 3) and [2xARC] (lane 4) resulted in high yield of the green fluorescent protein (GFP). Artificial translational enhancer [3xARC] provided high level of the AFPh synthesis (lane 2) compared to the AFPh translation when the [pl] was used as a 5′-UTR (lane 1).
Then, we analyzed products of translation of the [pl]-[AFPh]-[3′-TMV], [3хARC]-[AFPh]-[3′-TMV] and [PVY]-[AFPh]-[3′-TMV] mRNAs by western blot analysis (Fig. 5). Monoclonal antibodies to AFPh detected major polypeptides in the lanes 1-3 that match in size with AFPh protein (70 kDa).
![Figure 5: Western blot analysis. AFPh polypeptides synthesized in the wheat germ cell-free system. M – protein marker (size marked in kDa on the left); 1 – AFPh translation products from the mRNA with the 5'-UTR [3xARC]; 2 – AFPh translation product from the mRNA with the control 5'-UTR [pl]; 3 – AFPh translation products from the mRNA with the 5'-UTR [PVY]; 4 – control in vitro translation without exogenic mRNAs.](https://www.biotech-asia.org/wp-content/uploads/2016/12/Vol_13_no4_Clon_Guls_fig5-150x150.jpg) |
Figure 5: Western blot analysis. AFPh polypeptides synthesized in the wheat germ cell-free system. M – protein marker (size marked in kDa on the left); 1 – AFPh translation products from the mRNA with the 5′-UTR [3xARC]; 2 – AFPh translation product from the mRNA with the control 5′-UTR [pl]; 3 – AFPh translation products from the mRNA with the 5′-UTR [PVY]; 4 – control in vitro translation without exogenic mRNAs. |
Conclusions
Obtained data shows the possibility of oncofetal AFPh protein synthesis in the wheat germ cell-free system.
It should be noted that the presence of the translational enhancers in mRNA significantly increased the yield of the synthesized protein. The highest yield was obtained using the 5′-UTR of the potato virus Y genomic RNA, although the artificial enhancer [3xARC] also significantly enhanced the translation of the AFPh mRNA.
Financial Support
This work was done with financial support from scientific project 0158/POF-14 “The development of biotechnological bases for the creation and monitoring of genetically modified plants with improved economically valuable traits” for 2015-2017 years of the Committee of Science of the Ministry of Education and Science of the Republic of Kazakhstan.
References
- Abelev, G.I. Alpha-fetoprotein: 25 years of study. Tumor Biol., 1989; 10: 63-74.
CrossRef - Torres, J.M., Laborda, J., Naval, J., Darracq, N., Calvo, M., Mishal, Z., Uriel, J. Expression of alpha-fetoprotein receptors by human T-lymphocytes during blastic transformation. Mol Immunol., 1989; 26: 851–857.
CrossRef - Ignateva, G.A., Toptygin, A.Yu., Seslavina, L.S., Sidorovich, I.G. Antitumor effect of the alpha-fetoprotein conjugated with ristomycin. I Natsionalnaya konferentsiya Rossiyskoy assotsiatsii allergologov i klinicheskikh immunologov “Sovremennye problemy allergologii, klinicheskoy immunologii i immunofarmakologii” [First National Conference of the Russian Association of Allergology and Clinical Immunology “Modern Problems of Allergology, Clinical Immunology and Immunopharmacology”]. Moscow, 1997; p. 269. (In Russian)
- Luzhkov, Yu.M., Moskaleva, E.Yu., Nakashyan, R. et al. Konyugaty biologicheski aktivnykh veshchestv s alfa-fetoproteinom, obladayushchie izbiratelnym deystviem po otnosheniyu k rakovym opukholyam, sposob ikh polucheniya (varianty) i farmatsevticheskaya kompozitsiya na ikh osnove [Active substances conjugated with alpha-fetoprotein possessing selective activity against cancerous tumors. Methods (variants) of production and pharmaceutical composition on their basis]. Patent RF, no. 2071351, 1997. (In Russian)
- Roshchin, E.M., Komov, D.V., Nechipay A.V. et al. The theoretical study and the results of the first clinical application of human alpha-fetoprotein (AFP) and the estrone–doxorubicin (ED) complex at patients with malignant tumors of the liver. Novoe v oncologii [New in oncology], 1997; 2: pp. 137–140. (In Russian)
- Severin, S.E., Feldman, N.B., Lutsenko, S.V. et al. Increasing of the doxorubicin’s antitumor activity by its targeted delivery to the target cells via protein vectors. Voprosy biologicheskoy, meditsinskoy i farmakologicheskoy khimii [Issues of biological, medical and pharmocological chemistry], 1999; 1: pp. 44–48.
- Tikhonov, A.V., Shcherbakov, V.M., Shutov, A.V. Sposoby polucheniya preparata dlya napravlennoy dostavki protivoopukholevykh lekarstv v rakovuyu kletku [Methods of the antitumor drug production for the targeted delivery into cancer cells]. Patent RF, no. 2026688, 1999. (In Russian)
- Mizejewski, G.J., MacColl, R. α-fetoprotein growth inhibitory peptides: potential leads for cancer therapeutics. Mol. Cancer Ther., 2003; 2: 1243‑1255.
- Hooper, D.C., Cohen, B.L., Ducas, D., Gronvik, K.O., Hoskin, D.W., Murgita, R.A. Selective inhibition of murine Tcell proliferative and lymphokine-activated natural killer cell function by alpha-fetoprotein. In: Biological Activities of Alpha Fetoprotein (Mizejewski GJ & Jacobson H, eds). Boca Raton: CRC Press, 1987; pp. 153‑167.
- Laborda, J., Naval, J., Allouche, M., Calvo, M., Geogoulias, V., Mishal, Z., Uriel, J. Specific uptake of alpha–fetoprotein by malignant human lymphoid cells. Int J Cancer., 1987; 40: 314‑318.
CrossRef - Semenkova, L.N., Dudich, E.I., Dudich, I.V. Induction of apoptosis in human hepatoma cells by alpha-fetoprotein. Tumor Biol., 1997; 18: 261‑273.
CrossRef - Dudich, E., Semenkova, L., Dudich, I., Gorbatova, E., Tochtamisheva, N., Tatulov, E., Nikolaeva, M., Sukhikh, G. Alpha-fetoprotein causes apoptosis in tumor cells via a pathway independent of CD95, TNFR1 and TNFR2 through activation of caspase-3-like proteases. Eur. J. Biochem., 1999; 266: 750‑761.
CrossRef - Thompson, С.В. Apoptosis in the pathogenesis and treatment of disease. Science, 1995; 267: 1456‑1462.
- Fujiwara, Т., Grimm, E.A., Mukhopadhyay, T., Cai, D.W., Owen‑Schaub, L.B., Roth, J.A. A retroviral wild type p53 expression vector penetrates human lung cancer spheroids and inhibits growth by including apoptosis. Cancer Res, 1993; 53: 4129‑4133.
- Posypanova, G.A., Kireeva, N.N., Makarov, V.A., Fattakhova, G.V., Pozmogova, G.E., Popova, O.N., Zabolotnev, D.V., Severin, S.E., Severin, E.S. Use of alpha-fetoprotein for targeted delivery of antisense oligonucleotides into tumor cells: comparison of two constructs. Voprosy biologicheskoy, meditsinskoy i farmakologicheskoy khimii [Issues of biological, medical and pharmocological chemistry], 2005; 3: pp. 15‑20. (In Russian)
- Sambrook, J., Russel, D.W. (ed): Molecular Cloning. A laboratory Manual, 3rd edn. New York: Cold Spring Harbor Laboratory Press, 2001; 1: 1.1.
- Gurevich, V.V., Pokrovskaya, I.D., Oburhova, T.A., Zozulya, S.A. Preparative in vitro mRNA synthesis using SP6 and T7 RNA polymerases. Analyt. Biochem., 1991; 195: 207‑213.
CrossRef - Johnston, F.B., Stern, H. Mass isolation of viable wheat embryo. Nature, 1957; 179: 160‑161.
CrossRef - Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 1972; 227: 680‑685.
CrossRef - Sambrook, J., Russel, D.W. (ed): Molecular Cloning. A laboratory Manual, 3rd edn. New York: Cold Spring Harbor Laboratory Press, 2001; 3: A8.52
- Harper, M.E., Dugaiczyk, A. Linkage of the evolutionarily-related serum albumin and alpha-fetoprotein genes within q11-22 of human chromosome 4. Am. J. Hum. Genet., 1983; 35: 565‑572.
- Sakai, M., Morinaga, T., Urano, Y., Watanabe, K., Wegmann, T.G., Tamaoki, T. The human alpha-fetoprotein gene. Sequence organization and the 5′ flanking region. J. Biol. Chem., 1985; 260: 5055-5060.
- Morinaga, T., Sakai, M., Wegmann, T.G., Tamaoki, T. Primary structures of human alpha-fetoprotein and its mRNA. Proc. Natl. Acad .Sci. USA., 1983; 80: 4604‑4608.
CrossRef - Akbergenov, R., Zhanybekova, S., Kryldakov, R., Zhigailov, A, Polimbetova, N., Hohn, T., Iskakov, B. ARC-1, a sequence element complementary to an internal 18S rRNA segment, enhances translation efficiency in plants when present in the leader or intercistronic region of mRNAs. Nucleic Acids Research, 2004; 32: 239‑247.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





