How to Cite | Publication History | PlumX Article Matrix
G. Dhanavathy and Sylvia Jayakumar
1Department of Biotechnology, School of Bioengineering,SRM University, Kattankulathur, Tamilnadu, India. 2Consultant Biostatistician, Benz academy, Chennai.
Corresponding Author E-mail: dhanavathy.g@ktr.srmuniv.ac.in
DOI : http://dx.doi.org/10.13005/bbra/2456
ABSTRACT: Diabetes mellitus (DM) is a chronic disease caused by inherited or acquired deficiency in insulin secretion and/or by decreased responsiveness of the organs to insulin, is currently one of the most costly and burdensome diseases and affects about 5% of the global population with diabetic complications. Traditional herbal medicine has been widely used for disease treatment and is recognized as an interesting alternative to conventional medicine. Even natural herbal drugs are toxic above a certain concentration and hence it is essential to find out the effective concentration of the drug. Hence the amount of this swertiamarin to be administered in diabetic rats is an important factor in treatment of diabetes mellitus. Thus the assessment of effective dosage of the drug for fixing the dose is the most essential step in deciding the optimum activity of the drug. Inorder to achieve the optimum dosage, acute and subchronic toxicity studies of swertiamarin in Wistar rats was performed.
KEYWORDS: Toxicity; Swertiamarin; diabetes mellitus
Download this article as:| Copy the following to cite this article: Dhanavathy G, Jayakumar S. Acute and Subchronic Toxicity Studies of Swertiamarin A lead Compound Isolated from Enicostemma Littorale.blume in wistar rats. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Dhanavathy G, Jayakumar S. Acute and Subchronic Toxicity Studies of Swertiamarin A lead Compound Isolated from Enicostemma Littorale.blume in wistar rats. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=21328 |
Introduction
Diabetes mellitus is one of the globally challenged chronic metabolic disorders characterized by hyperglycemia arising as a consequence of deficiency in pancreatic insulin production and or in insulin action on peripheral tissues.1-3 The prevalence of diabetes worldwide for, all range of age-groups was estimated to be 2.8% in 2000 and expected to rise to 4.4% in 2030.4 Diabetes mellitus is associated with risk factors of microvascular and macrovascular complications such as nephropathy, neuropathy, retinopathy and cardio vascular diseases and the basis of abnormalities in carbohydrate, fat and protein metabolism in diabetes resulting from diminished tissue responses to insulin action.5 Diabetes mellitus is rapidly spreading in developing countries as a result of westernization of lifestyle and food habits.6
Therapeutic remedies for diabetes are diet, physical activity, synthetic oral hypoglycemic drugs and insulin therapy. Treatment with synthetic drugs causes side effects such as gastrointestinal disturbances, hypoglycemia, rise in hepatic enzymes, hematological diseases.7
Enicostemma littorale Blume is one among the several antidiabetic perennial herbs belonging to the family Gentianaceae. The plant is also known as Chotachirayata in Hindi, Mamejavo in Gujarati, Nagajivha in Bengal and Vellarugu or Vallari in Tamil.8
Swertiamarin from Enicostemma littorale Blume possess various pharmacological activities, such as neuropathy, antinociceptive, antimicrobial, antihelminthic, antihyperlipidaemic, antioxidant, antiatherogenic, antiulcer, anti-inflammatory, antitumor, hepatoprotective and antiedematogenic.9
In spite of a long and more traditional utilization of E.littorale and some reports on the hypoglycemic, hypolipidaemic, hepatoprotective, antioxidant action of the compound swertiamarin, no systematic studies on cytoprotective activity of swertiamarin have been carried.
Even natural herbal drugs are toxic above a certain concentration and hence it is essential to find out the effective concentration of the drug. Hence the amount of this swertiamarin to be administered in diabetic rats is an important factor in treatment of diabetes mellitus. Thus the assessment of effective dosage of the drug for fixing the dose is the most essential step in deciding the optimum activity of the drug. Inorder to confirm the optimum dosage, acute and subchronic toxicity studies of swertiamarin in Wistar rats was performed.
Acute and Subchronic toxicity studies
Medicinal plants, as a pure compound, offers unlimited and valuable path for the discovery of novel drugs. However, there is very precise proof available concerning the possible toxicity that medicinal plants may cause to the consumers of plant based drug therapy.10 Based on their experience on long-term exposure by humans, plants used in traditional medicine are expected to have low toxicity compared to synthetic drugs. In contrast, the latest surveys have revealed that many medicinal plants applied in traditional medicine showed adverse effects on the consumers.11,12 This raises anxiety about the potential toxic effects resulting from either the short-term or long-term use of such medicinal plants as therapeutic drugs. Therefore, evaluating the toxicological effects of any lead compounds derived from medicinal plants intended to be used in animals or humans is a crucial and authenticating part of its assessment for potential toxic effects.13 Hence the aim of the present study was to evaluate the comprehensive in vivo acute and subchronic oral toxicity of swertiamarin in Wistar rats by assessing the hematological and biochemical parameters.
Materials and Methods
Acute and Subchronic toxicity studies
Selection and Maintenance of Experimental Animals for Acute and Subchronic toxicity studies
Acute and subchronic toxicity studies were carried out on male Wistar strain rats weighing about 190–250 g bred in the laboratory of king’s institute for preventive medicine, Guindy, Chennai, Tamil Nadu, India. All the animals were maintained under laboratory conditions of temperature (22±2 ◦c), humidity (45±5%) and 12 h day: 12 h night cycle and were provided with commercially available rat pellet diet purchased from Sai enterprises, Chennai, India (60% carbohydrate, 20% protein, 10% vitamin and mineral mix, 5% fat and 5% cellulose) and water ad libitum. All the animals were acclimatized to normal laboratory conditions for 7 days before commencement of the experiment. The experimental protocols were performed in accordance by the Institutional Animal Ethical committee for the purpose of Control and Supervision of Experiments on Animals (CPCSEA), under the Ministry of animal welfare division, Government of India, New Delhi, India.14
Acute toxicity study
Acute toxicity test was performed according to the Organization of Economic Cooperation and Development (OECD) guideline 420 for testing of chemicals and drugs.15 Thirty six male Wistar albino rats, weighing 190-250g were used for the acute toxicity study. They were randomly divided into one normal control group and five treated groups, containing six animals in each group and were on standard normal pellet diet provided with water ad libitum. They were allowed to acclimatize for seven days to the laboratory condition prior to the experiment. The treated group received orally varying doses of swertiamarin (5, 50, 300, 500, 2000mg/ Kg body weight) isolated from E.littorale which was dissolved in water were fed to the rats at the rate of 1.0ml /rat/day to different sets of animals for 15days.Control group animals treated with water served as control. The rats were weighed and body weight was recorded. Rats were continuously observed for a period of 15 days to detect any changes in autonomic or behavioral responses. The rats were weighed and body weight was recorded. Any mortality during the experimentation period of 15 days was also recorded. The percentage in mortality in each group was noted.16
Subchronic oral toxicity study
Inorder to find out the effective dosage of swertiamarin, subchronic toxicity studies were carried out. Subchronic toxicity test was performed according to the Organization of Economic Cooperation and Development (OECD) guideline 420 for testing of chemicals.17 Male Wistar rats were randomly assigned into six groups: Normal control group and five treatment groups (𝑛 = 6). Treatment group received swertiamarin of oral dosage range of 5, 50, 100,300 and 500 mg/kg body weight and the control group received only water at a rate of 1ml/ rat/day for 50 days, once daily.18 The drug was freshly prepared with water on daily basis.
Experimental Design for subchronic oral toxicity study
Rats were divided into six groups of six rats in each group:Group I: Normal control, Group II: Swertiamarin (5 mg/kg),Group III: Swertiamarin (50 mg/kg), Group IV: Swertiamarin (100 mg/kg), Group V: Swertiamarin (300 mg/kg),Group VI: Swertiamarin (500 mg/kg) daily.
Body Weight Assessment
The body weight of each rat was assessed and recorded using a sensitive balance during the acclimatization period, once before commencement of drug dosing, once weekly during the drug dosing period and once on the day of sacrifice. The rats were weighed and the body weight was recorded to analyze the average increase in body weight.19
Mortality and clinical observation
During the 50 days dosing period, all the animals were observed daily for clinical signs and mortality patterns once before drug dosing, immediately after drug dosing and up to 4 hour after drug dosing.20,21 Any mortality during the experimentation period of 50 days was also recorded. The percentage in mortality in each group was noted.22
Haematology and Clinical Biochemical Analysis
At the end of the experimental study period (50th day), the rats were anesthetized through intraperitoneal injection of ketamine (60mg/kg) and xylazine (7.5mg/kg). Blood samples were collected via retro-orbital puncture into two different tubes of nonheparinized and EDTA-containing for both biochemical and haematological analyses, respectively. Haematological parameters include Hb, RBC, WBC and platelet count.23 Biochemical parameters include glucose, urea, uric acid, Creatinine in serum of control and experimental rats.23 Haematological and biochemical analyses were performed by using auto analyzers and standard kits.
Histopathology study
After sacrificing the rats, parts of the organs like liver, kidney, pancreas, were collected for histological studies. The tissues were washed in ice-cold normal saline and fixed immediately in 10% formalin for a period of at least 24 h, dehydrated with alcohol, embedded in paraffin, cut into sections, and stained with haematoxylin-eosin dye for photomicroscopic observation. The microscopic features of the organs of treated rats were compared with the control group.24,25
Statistical Analysis
The results were expressed as mean±S.D. The statistical significance was evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test using the SPSS/11.5 software. Values were considered statistically significant at p ≤ 0.05.
Results
Acute and subchronic toxicity studies
Acute oral toxicity
In the acute toxicity study, the rats were treated with different doses of swertiamarin orally from the range of 5 – 2000 mg/kg body weight which did not produce significant signs of toxicity, behavioral responses, physiological changes, physical observations (skin, fur, eyes mucous membrane, behavior patterns, tremors, salivation, and diarrhea of the rats) and mortality in the test groups when compared to the controls, observed during the entire 15 days of the acute toxicity experimental study period. There was neither mortality observed at the tested dose nor any weight loss in this study group rats.
Subchronic toxicity study
Mortality and clinical signs
In subchronic toxicity study, oral dosage range of swertiamarin was 5mg, 50 mg, 100mg, 300mg and 500mg/kg. All the animals were free of any intoxicating signs and physical changes even in the highest dose (500 mg/kg) group throughout the drug dosing period of 50 days. No mortality and no clinical signs in any group were observed throughout the experimental period.
Weekly body weight
All rats showed significant increase in body weight compared to their initial values. However there was no significant difference between the different treatment groups and the control, indicating that the drug did not possessed any potent effect on the body weight, which is used to assess the response to the therapy of drug (Figure 1.1). No signs of toxicity or mortality were observed during the whole 50 days experimental period.
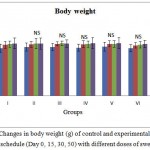 |
Figure 1.1 Changes in body weight (g) of control and experimental rats during the treatment schedule (Day 0, 15, 30, 50) with different doses of swertiamarin
|
Values are expressed as mean ± SD (n = 6 for each group)
Group I: Normal control, Group II: Normal + SM (5 mg/kg), Group III: Normal + SM (50 mg/kg), Group IV: Normal + SM (100 mg/kg), Group V: Normal + SM (300 mg/kg), Group VI: Normal + SM (500 mg/kg)
NS – not significant, Comparison between groups and control
Haematology Parameter Analysis
The effects of subchronic administration of swertiamarin on haematological parameters were studied. Most haematology measures (haemoglobin (Hb), red blood cells (RBC), white blood cells (WBC) and platelet count in treated rats were not significantly different from the controls, with the exception of border line marginal variations in certain parameters (Figure 1.2 & 1.3 respectively). From the present study it was clearly seen that there was no significant change in the haematological parameters in the swertiamarin treated group compared to the normal control group.
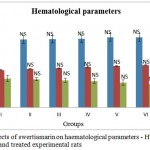 |
Figure 1,2: Effects of swertiamarin on haematological parameters – Hb, RBC, WBC of the control and treated experimental rats
|
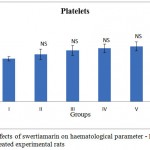 |
Figure 1,3: Effects of swertiamarin on haematological parameter – Platelets of the control and treated experimental rats |
Values are expressed as mean ± SD (n = 6 for each group)
Group I: Normal control, Group II: Normal + SM (5 mg/kg), Group III: Normal + SM (50 mg/kg), Group IV: Normal + SM (100 mg/kg), Group V: Normal + SM (300 mg/kg), Group VI: Normal + SM (500 mg/kg)
NS– not significant, Comparison between groups and control
Analysis of biochemical parameters
Figure 1.4 shows the effect of the swertiamarin on biochemical parameters including glucose, urea, uric acid, creatinine in serum of control and experimental rats. The kidney function parameters (urea, creatinine, and uric acid) did not reveal any relevant changes following administration of swertiamarin These results clearly exhibited that the swertiamarin at different levels tested did not produce any considerable changes in the levels of the different parameters tested.
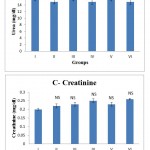 |
Figure 1.4: A, B, C, D – Effect of swertiamarin on serum biochemical parameters in control and experimental rats
|
Values are expressed as mean ± SD (n = 6 for each group).
Group I: Normal control, Group II: Normal + SM (5 mg/kg), Group III: Normal + SM (50 mg/kg), Group IV: Normal + SM (100 mg/kg), Group V: Normal + SM (300 mg/kg), Group VI: Normal + SM (500 mg/kg)
NS – not significant, Comparison between groups and control
Histopathological studies
Histopathological evaluations of the various vital organs, liver, kidney and pancreas, were stained with haematoxylin and eosin by light microscopy of both swertiamarin treated and control group rats showed normal structure and absence of any pathological lesions which confirmed that there was no significant difference between the swertiamarin treated and control rats (Figure 1.5). This authenticates and proves the non toxic nature of swertiamarin and its ability to be used as hypoglycemic drug.
The LD50 of swertiamarin was therefore estimated to be more than 500mg/kg and thus the dosage range is safe with efficient bioactivity. Swertiamarin, effective dosage studies from subchronic toxicity studies clearly revealed that the highest dosage level is 500 mg/kg and hence the final swertiamarin drug dosage for all the further studies was fixed as swertiamarin of 15, 25, 50 mg/kg body weight.
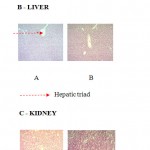 |
Figure 1.5: Histological photomicrographs of SM (300 mg/kg) on various rat organs A -pancreas, B – liver, C – kidney sections of Wistar rats in subchronic toxicity study (H&E)
|
A – Normal control, B – SM (500 mg/kg) (Magnification x 100)
Discussion
Diabetes mellitus is a chronic metabolic disorder of absolute insulin deficiency resulting from autoimmune destruction of insulin producing β-cells of the pancreas by streptozotocin. Streptozotocin is a potent diabetogenic agent causing pancreatic β-cell cytotoxicity, inducing diabetes mellitus.26 STZ damages β-cells by producing superoxide radicals inside β-cells which are formed by xanthine oxidase or is taken up by pancreatic β-cells via the glucose transporter (GLUT2) and causes alkylation of DNA,32 thereby damaging the pancreatic β-cells causing abnormalities in carbohydrate and lipid metabolism and it is markedly increased in adult populations.26 STZ diabetic model is one of the most widely accepted and utilized method to induce diabetes mellitus in animals.
Inorder to control hyperglycemia, dyslipidemia, insulin resistance and other diabetic complications, many synthetic drugs were used which caused adverse side effects which forced the search of safer and potent antidiabetic drugs.27 The present study on the antidiabetic efficacy of swertiamarin was carried out, as there have been reports that various synthetic hypoglycemic drugs available in market, like metformin, glimepiride, nateglinide have numerous side effects. Metformin cause weight loss and gastrointestinal complications like nausea, abdominal pain, diarrhea, severe hypoglycaemia.28,29 Sulphonyl urea drugs like glipizide, gliclazide, glibornuride, gliquidone, glisoxepide, glyclopyramide and glibenclamide causes severe hypoglycemia and weight gain.30 Therefore, to overcome these adverse effects there is an urgent need for the development of hypoglycemic drugs from natural resources such as medicinal plants.31
In recent years many plants have been screened for lowering blood glucose and glucose homeostasis. However plant based herbal drugs showed adverse toxic side effects if the concentration of drug administered is not standardized before oral administration. Inorder to overcome this challenge the effective dose of drug is fixed by performing acute and subchronic toxicity studies of swertiamarin on Wistar rat models.32
Acute toxicity studies
Natural products from medicinal plants have formed the basic foundation for the treatment of various ailments for decades.33,34 Inorder to achieve discovery of new pharmacological active compound, screening natural products for pharmacological activity, evaluation and assessment of the toxic characteristic of isolated lead compound (LD50) are the beginning steps. Inspite of the valuable, beneficial, pharmacological effects of swertiamarin, a deep knowledge about the toxicology of this famous drug is lacking. Hence the current study was performed to evaluate the acute and subchronic toxicity of swertiamarin in animal models (rats). Data from the acute toxicity study may (1) provide initial ground information on the mode of toxic action of a drug, (2) help in dose determination in animal studies and (3) help determine LD50 values that provide many benefits to arrive at an effective dose of a new lead drug compound.35 Hence, if a high dose (e.g., 2,000 mg/kg) is found to be nontoxic and survivable, no further acute testing is required and will not be conducted.36 In this study, swertiamarin at a dose of 2000mg/kg had no adverse effect on the tested rats up to 15 days of observation supported by previous reports.37 There was no significant change in the body weight, toxicity and no mortality was observed in the swertiamarin treated test groups when compared to the control groups during the entire study period which may indicate that the drug is safe and nontoxic.38 Therefore, this study indicates that swertiamarin does not cause acute toxicity effects at the dose tested and with an LD50 value greater than 2000 mg/kg.
Subchronic toxicity studies
Subchronic toxicity studies, gives information on target organ toxicity and are designed mainly to identify no observable adverse side effect level.36 Subchronic evaluation mainly enhances to determine appropriate dose regimens for longer term studies. Swertiamarin was evaluated in rats at doses of 5, 50, 100, 300, 500mg/kg/day for 50 days in subchronic toxicity studies. Administration of swertiamarin for 50 days produced no clinical signs of toxicity or mortality. The swertiamarin treated rats did not also show any significant alteration in water or food consumption. Loss of appetite is often synonymous with body weight loss due to disturbances in carbohydrate, protein or fat metabolisms.39 In this scenario, drug is converted to a toxic end product, which interferes with gastric function and decreased efficiency in food conversion.40 In this study the food and water were well-accepted by the rats treated with swertiamarin suggesting that the drug did not cause any alterations in carbohydrate, protein or fat metabolism in these experimental animals. Therefore, swertiamarin can be considered as non-toxic up to the tested experimental dose.
The body weight changes serve as a sensitive and authenticated indicator of the general health status of animals.41 However, the observed increase in body weight could be attributed to the nutritive components in the drug swertiamarin.42,43 Interestingly, it was noted that the dose 500 mg/kg/day further increased the body weight in rats after the 50th day of treatment with swertiamarin.
The serum haematology and clinical biochemistry analyses were performed to assess the possible alterations in both hepatic and renal functions influenced by swertiamarin. Liver and kidney function analysis is very important in the toxicity evaluation of the drug swertiamarin as they are both necessary for the healthy survival of any living organism.44 Renal dysfunction can be monitored by concurrent analysis of urea, creatinine and uric acid and their normal levels reflect the normal renal function.45 In the current study, changes in serum urea, creatinine, and uric acid levels in swertiamarin treated groups showed non-significant differences indicating a normal and healthy renal function.
Evaluation of haematological parameters can be used to determine the degree of the deleterious effect of swertiamarin on the blood of an animal.46 These analyses are relevant to risk evaluation as changes in the haematological system have higher predictive value for human toxicity when the data are translated from the animal studies.47 A haemogram was undertaken for all the swertiamarin treated and control groups and the results show no significant deleterious effects. The nonsignificant effect of the swertiamarin on red blood cells, Hb, and platelets indicates that the swertiamarin does not affect the erythropoiesis, morphology, or osmotic fragility of the red blood cells.48 A normal haematological profile of swertiamarin treated groups which further justified the non-toxic nature of swertiamarin. The macroscopic examinations of the organs of rats treated with various doses of swertiamarin did not show any changes in colour compared with control group rat’s organs. Hypertrophy of any organs is first hand indication of toxicity of chemical or biological substance. However, no hypertrophy of organs was observed amongst all the groups studied.
The microscopic histopathological examination also revealed that none of the organs from the swertiamarin treated rats showed any alteration in cell structure when viewed under the light microscope. No pathologies were recorded in the histological sections of the vital organs including liver, kidney and pancreas of the drug treated and control group. Generally, any damage to the parenchymal hepatic cells results in elevations of liver biomarkers in the blood.49 Thus, the non-significant increases observed in liver biomarker activities strongly suggest that the subchronic administration of swertiamarin did not alter the hepatocytes and its metabolic activities of the rats as observed in the histopathology observations of liver tissue. There was also no significant increase in urea, creatinine, and uric acid in the subchronic administration of swertiamarin when compared to the control group. Any rise in urea, creatinine and uric acid levels is indicative of marked damage to functional nephrons.50 These findings were further confirmed and proved by histopathological observations of the kidney tissues in this study.
Conclusion
The results of our study authenticated that oral administration of swertiamarin did not alter any parameters with special focus on histopathology which did not exhibit any lesions or abnormalities, which prove that no toxic adverse effects of the drug and further support the non-toxic nature of swertiamarin at the fixed dosage of 15, 25, 50 mg/kg. Further studies of the swertiamarin drug at the fixed dosage (15, 25, 50 mg/kg) by biochemical parameters enhance its pharmacological studies and nature to authenticate it as an potent antidiabetic lead drug.
Acknowledgements
The author thanks the institutional supporting rendered by the SRM University throughout the research activities.
Reference
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30:42–47.
CrossRef - Shalam M. D., Harish M. S., Farhana S. A. Prevention of dexamethasone and fructose-induced insulin resistance in rats by SH01D a herbal preparation. Indian J. Pharmacol. 2006;38(6):419–422.
CrossRef - World Health Organization,Definition diagnosis and classification of diabetes mellitus and its complications. In: report of WHO consultation., Part 1: diagnosis and classification of diabetes mellitus., World Health Organization, Geneva. 1999.
- Aziz M. T. A., El-Asmar M. F., Rezq A. M., Mahfouz S. M., Wassef M. A., Fouad H. H., Ahmed H. H., Taha F. M. The effect of a novel curcumin derivative on pancreatic islet regeneration in experimental type-1 diabetes in rats (long term study). Diabetol Metab Syndr. 2013;5(75).
CrossRef - Randhir S., Navpreet K., Lalit K., Girish G. K. Management of diabetic complications: A chemical constituents based approach. J. Ethnopharmacol. 2013;150(1):51–70,
CrossRef - Aziz M. T. A., El-Asmar M. F., Rezq A. M., Mahfouz S. M., Wassef M. A., Fouad H. H., Ahmed H. H., Taha F. M. The effect of a novel curcumin derivative on pancreatic islet regeneration in experimental type-1 diabetes in rats (long term study). Diabetol Metab Syndr. 2013;5(75).
CrossRef - Kumar R., Arora V., Ram V., Bhandari A., Vyas P. Hypoglycemic and hypolipidemic effect of Allopolyherbal formulations in streptozotocin induced diabetes mellitus in rats. Int J. Diabetes Mellitus. 2011;3(1):45-50.
CrossRef - Kirtikar K. R., Basu B. D., Singh B., Pal M. S. Indian medicinal plants. Dehra Dun. 1935;3(2):1655–1656.
- Sadique J., Chandra T., Thenmozhi V and Elango V. The anti-inflammatory activity Enicostemma littorale and mullogo cerviana. Biochem Med. Metab. Biol. 1987;37:167-176.
CrossRef - Dias F. D. L and Takahashi C. S. Cytogenetic evaluation of aqueous extracts of the medicinal plants Alpinia mutans rose (Zingerberaceae) and Pogostemumhyneanus benth (Labitae) on Wistar rats and Allium cepa (Liliaceae) root tip cells. Brazilian Journal of Genetics. 1994;17(2):175–180.
- Ertekin V., Selimoˇglu M. A and Altinkaynak S. Acombination of unusual presentations of Datura stramonium intoxication in a child: rhabdomyolysis and fulminant hepatitius. Journal of Emergency Medicine. 2005;28(2):227–228.
CrossRef - Koduru S., Grierson D. S and Afolayan A. J. Antimicrobial activity of Solanum aculeastrum. Pharmaceutical Biology. 2006;44(4):283–286.
CrossRef - Yuet K. P., Darah I.,Chen Y.,Sreeramanan S and Sasidharan S. Acute and sub chronic toxicity study of Euphorbia hirta methanol extract in rats. BioMed Research International. 2013;1-14.
CrossRef - Eliza J., Daisy P., Ignacimuthu S., Duraipandiyan V. Normoglycemic and hypolipidemic effect of costunolide isolated from Costus speciosus (Koen ex. Retz.)Sm. in streptozotocin-induced diabetic rats.Chem Biol Interact. 2009;179:329–334.
CrossRef - Organization of Economic Co-operation and Development (OECD), The OECD Guideline for Testing of Chemicals 420, Acute Oral Toxicity- Fixed Dose Procedure, OECD, Paris, France. 2001.
- Stolar M. W., Hoogwerf B.J., Gorshow S. M. Managing type 2 diabetes going beyond glycemic control. J Manag Care Pharm. 2008;14:2-19.
CrossRef - Organization of Economic Co-operation and Development (OECD), The OECD Guideline for Testing of Chemicals: 408, “Subchronic Oral Toxicity-Rodent: 90-Day Study, OECD, Paris, France. 1998.
- Jaishree V., Badami S., Kumar M. R., Tamizhmani T. Antinociceptive activity of swertiamarin isolated from Enicostemma axillare. Phytomedicine. 2009;16:227–232.
- Sathya M., Kokilavani R., Teepa K. S. A. Acute and subacute toxicity studies of ethanolic extract of Acalypha indica Linn in male Wistar albino rats. Asian Journal of Pharmaceutical and Clinical Research. 2012;5(1).
- Reasner C. A. Reducing cardiovascular complications of type 2 diabetes by targeting multiple risk factors.J Cardiovasc Pharmacol. 2008;52:136–44.
CrossRef - Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas.Physiol Res. 2001;50:536– 546.
- Sathya M., Kokilavani R., Teepa K. S. A. Acute and subacute toxicity studies of ethanolic extract of Acalypha indica Linn in male Wistar albino rats. Asian Journal of Pharmaceutical and Clinical Research. 2012;5(1).
- Kjeldsberg C. R. Princípios do exame hematológico. Wintrobehematologia clínica. 1998;1:7-42.
- Abdollahi M., Zuki A. B., Goh Y. M. Effects of Momordica charantia on pancreatic histopathological changes associated with streptozotocin-induced diabetes in neonatal rats. Histol Histopathol. 2011;26:13-21.
- Ezeonwumelu J. O. C., Julius A. K., Muhoho C. N., Ajayi A. M., Oyewale A. A.,Tanayen J. K. Biochemical and histological studies of aqueous extract of Bidens pilosa leaves from Ugandan Rift Valley in Rats. British Journal of Pharmacology and Toxicology. 2011;2:302–209.
- Cooke D. W., Plotnick L. Type 1 diabetes mellitus in pediatrics. Pediatr. Rev. 2008;29:374–384.
CrossRef - Ahmed D., Kumar V., Verma A., Gupta S. P., Kumar H., Dhingra V.,Mishra V and Sharma M. Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia Lebbeck Benth. stem bark (ALEx) on Streptozotocin induced diabetic rats. BMC Complementary and Alternative Medicine. 2014;14:243.
CrossRef - Grover, JK., Rathi, SS., Vats, V., “Preliminary study of Fresh juice of Benincasa hispida on morphine addiction in mice,” Fitoterapia., 71(6), pp.707–709, 2000.
CrossRef - Garber A. J., Duncan T. G., Goodman A. M. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med. 1997;103:491-7.
CrossRef - Stolar M. W., Hoogwerf B. J., Gorshow S. M. Managing type 2 diabetes going beyond glycemic control. J Manag Care Pharm. 2008;14:2-19.
CrossRef - Abdollahi M., Zuki A. B., Goh Y. M. Effects of Momordica charantia on pancreatic histopathological changes associated with streptozotocin-induced diabetes in neonatal rats. Histol Histopathol. 2011;26:13-21.
- Kulkarni S. K. Hand Book of Experimental Pharma- cology. Vallabh Prakashan., New Delhi, India. 1999;117.
- Patwardhan B., Ashok D. B and Chorghade M. V. Ayurveda and natural products drug discovery.Current Science. 2004;86( 6):25.
- Ridtitid W., Sae-Wong C., Reanmongkol W and Wongnawa M. Antinociceptive activity of the methanolic extract of Kaempferia galanga in experimental animals. Journal of Ethnopharmacology. 2008;118(2):225-230.
CrossRef - Ukwuani A. N., Abubakar M. G., Hassan S. W and Agaie B. M. Toxicological studies of hydromethanolic leaves extract of Grewia crenata. International Journal of Pharmaceutical Science and Drug Research. 2012;4:245-249.
- National Research Council (NRC), Toxicity testing for assessing environmental agents, Interim Report, National Academies Press., Washington, DC, USA. 2006.
- Kennedy G. L., Ferenz R. L and Burgess B. A. Estimation of acute oral toxicity in rats by determination of the approximate lethal dose rather than the LD50. Journal of Applied Toxicology. 1986;6(3):145-148.
CrossRef - Roopashree T. S., Raman D., Rani R. H. S and Narendra C. Acute oral toxicity studies of antipsoriatic herbal mixture comprising of aqueous extracts of Calendula officinalis, Momordica charantia, Cassia tora and Azadirachta indica seed oil. Thai Journal of Pharmaceutical Sciences. 2009;33(2-3):74-83.
- Klaassen C. D. Casarett and Doull’s Toxicology The Basic Science of Poisons, McGraw-Hill Press., New York, NY, USA.
- Chokshi D. Subchronic oral toxicity of a standardized white kidney bean (Phaseolus vulgaris) extract in rats. Food and Chemical Toxicology. 2007;45(1):32-40.
CrossRef - Hilaly J. E., Israili Z. H and Lyoussi B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. Journal of Ethnopharmacology. 2004;91(1):43–50.
CrossRef - Ezeonwumelu J. O. C., Julius A. K., Muhoho C. N., Ajayi A. M., Oyewale A. A.,Tanayen J. K. Biochemical and histological studies of aqueous extract of Bidens pilosa leaves from Ugandan Rift Valley in Rats. British Journal of Pharmacology and Toxicology. 2011;2:302–209.
- Duke J. A. The Green Pharmacy: New Discoveries in Herbal Remedies for Common Diseases and Conditions from the World’s Foremost Authorities on Healing Herbs. St Martins Press. 1997.
- Olorunnisola O. S., Bradley G and Afolayan A. J. Acute and sub-chronic toxicity studies of methanolic extract of Tulbaghia violacea rhizomes inWistar rats. African Journal of Biotechnology. 2012;11:14934–14940.
- Davis M. E and Bredt N. D. Renal methods for toxicity, in Principles and Methods of Toxicology Raven Press, New York, NY, USA. 1994.
- Yakubu M. T., Akanji M. A and Oladiji A. T. Haematological evaluation in male albino rats following chronic administration of aqueous extract of Fadogia agrestis stem. Pharmacognosy. 2007;3:34–38.
- Olson H., Betton G., Robinson D. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regulatory Toxicology and Pharmacology. 2000;32(1):56- 67.
CrossRef - Guyton A. C and Hall J. E. Textbook ofMedical Physiology W. B Saunder., Philadelphia, Pa, USA. 2000.
- Slichter S. J. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transfusion Medicine. 2004;18(3):153-167.
- Lameire N., Biesen W. V and Vanholder R. Acute renal failure. The Lancet. 9457;365:417-430.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






