Manuscript accepted on : 17 February 2017
Published online on: --
Plagiarism Check: Yes
Antifungal Effect of Copper and Copper Oxide Nanoparticles Against Penicillium on Orange Fruit
Hamideh Eslami Chalandar1, Hamid Reza Ghorbani2, Hosein Attar1 and Seyed Abolhasan Alavi1
1Department of Chemical Engineering, Science and Research branch, Islamic Azad University, Tehran, Iran.
2Department of Chemical Engineering, Qaemshahr branch, Islamic Azad University, Qaemshahr, Iran.
Corresponding Author E-mail: hamidghorbani6@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2445
ABSTRACT: Mold green and blue are most important disease of Citrus fruits after crop that it is created by fungus Penicillium italicum and Penicillium digitatum. In this study, copper and copper oxide nanoparticles synthesized by chemical reduction and precipitation methods and characterized by X-ray diffraction, FTIR, SEM and TEM. Also, the anti-fungal properties of synthesized nanoparticles were tasted against Penicillium on Orange fruit by Disc-Diffusion method. The antifungal properties of nanoparticles were studied by the effect of different concentrations of nanoparticles. The results show that anti-fungal properties also increased by increasing the concentration of nanoparticles to 15%.
KEYWORDS: Copper nanoparticles; Copper oxide nanoparticles; fungus Penicillium; anti-fungal properties
Download this article as:| Copy the following to cite this article: Chalandar H. E, Ghorbani H. R, Attar H, Alavi S. A. Antifungal Effect of Copper and Copper Oxide Nanoparticles Against Penicillium on Orange Fruit. Biosci Biotechnol Res Asia 2017;14(1) |
| Copy the following to cite this URL: Chalandar H. E, Ghorbani H. R, Attar H, Alavi S. A. Antifungal Effect of Copper and Copper Oxide Nanoparticles Against Penicillium on Orange Fruit. Biosci Biotechnol Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22021 |
Introduction
Copper nanoparticles have many applications in various industries, including the medical and pharmaceutical, chemical engineering, food industry, textile, paper and wood processing industry, electrical industry, cosmetic industry and agriculture. Also used as catalysts,1 lubricants 2 and nano fluids used.3 Copper has chemical and physical properties. Physical properties for copper include the copper metal bonds are relatively weak and lacks a covalent feature that it is descriptions of low hardness and high flexibility of the copper single crystals. When other metals are copper against galvanic corrosion will occur. Pure copper has red-orange color but, it when exposed to air is the opaque reddish found.4 Copper chemical properties include no reaction with water but, it makes black copper oxide layer by reaction with atmospheric oxygen.5 Copper and copper oxide nanoparticles synthesis by chemical, physical and biological methods. Antibacterial activity of copper and silver nanoparticles was examined by E.coli and Bacillus subtilis bacterial strains.6 Copper oxide biosynthesized with Streptomyces development of antimicrobial fabrics in hospitals can be useful to prevent or minimize infection by pathogenic bacteria.7 Copper and copper oxide nanoparticles synthesized by Morganella bacteria.8 As well as keeping food fresh for langer, enclaspes high cost in year. Copper and copper oxide with antimicrobial and antifungal effect can be sutuble factor for packing food to delaying the growth of germ and fungi. Multiple causative factors can be colonization, such as bacterial cell growth and the formation of intense microbial biofilm matrix, the bacteria is resistant to the host safety system, which is composed of nanoparticles of these factors prevent the host safety defense microbes against. Nano materials based on metal ions, has a wide cytotoxicity activity against bacteria, fungi and viruses are active. Nano-materials and Nano-metallic materials in particular due to their surface change and surface to volume ratio, enzymes and DNA of microorganisms with electron balance between electron donor groups such as thiol, carboxyl, amide, imidazole and hydroxyl disabled. Along with the rapid development of human life, it is inevitable to control harmful microorganisms.10,11 In the present study, copper and copper oxide nanoparticles were synthesized by chemical reduction and precipitation methods and their anti-fungal activities were studied by Disc-Diffusion method. For specify characterization, it was used X-ray diffraction (XRD), Transmission Electron Microscopy (TEM) and UV-Visible Spectrophotometer.
Experimental
Materials
All the chemicals used in the experiment were of analytical grade. Copper sulfate pentahydrate CuSO4. 5H2O (0.1M), Starch (C6H10O5)n (1.2%), Ascorbic acid C6H8O6 (0.2M), Sodium hydroxide NaOH (1M), copper acetate and absolute ethanol were purchased from Merck. De-ionized water was used for all the experiment.
Synthesis of Cu nanoparticles
The Cu nanoparticles were synthesized by chemical reduction process using copper (II) sulfate pentahydrate as Precursor salt and starch as capping agent. The preparation method starts with addition of 0.1 M copper (II) sulfate pentahydrate solution into 120 mL of starch (1.2 %) solution with vigorous stirring for 30 min. In the second step, 50 mL of 0.2 M ascorbic acid solution is added to synthesis solution under continuous rapid stirring. Subsequently, 30 mL of 1 M sodium hydroxide solution was slowly added to the prepared solution with constant stirring and heating at 80 0C for 2 h. The color of the solution turned yellow to ocher (Fig. 1). After the completion of reaction, the solution was taken from the heat and allowed to settle overnight and the supernatant solution was then discarded cautiously. The precipitates were separated from the solution by filtration and washed with deionized water and ethanol for three times to take out the excessive starch bound with the nanoparticles. Ocher color precipitates (Fig. 1) obtained are dried in oven at 60 0C for 4 h. The prepared nanoparticles were characterized by XRD, TEM and UV-Visible.
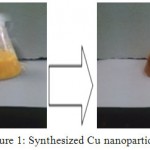 |
Figure 1: Synthesized Cu nanoparticles
|
Synthesis of CuO nanoparticles
The CuO nanoparticles were synthesized by precipitation process using Copper (II) acetate, sodium hydroxide and absolute ethanol were purchased from Merck. In a typical synthesis, 2.0 g copper acetate was dissolved in 80 mL absolute ethanol, and the mixture was stirred for 30 min, followed by addition of 0.8 g sodium hydroxide. Then the whole solution was transferred into a Teflon lined stainless steel autoclave, sealed, and maintained at 120 ◦C for 2 h. After cooling to room temperature, a black product was isolated by centrifugation, and washed several times with absolute ethanol and pure water (Fig. 2). Finally, the product was dried at 60 ◦C in air for 6 h. The prepared nanoparticles were characterized by XRD, TEM and UV-Visible.
 |
Figure 2: Synthesized CuO nanoparticles
|
Characterization
The prepared nanoparticles were characterized by XRD, TEM and UV-Visible. Structural characterization of nanoparticles was analyzed by X-ray diffraction. The scanning range of 2Ɵ was between 200 and 800. Sample preparation for XRD was quite easy as the nanoparticles were in a powder form. UV-Vis spectrophotometer obtained in the wavelength range 300-800 nm to investigate the optical properties of nanoparticles. TEM obtained to determine the morphology and particle size.
Anti-fungal properties of nanoparticles by Disc-Diffusion method
First, the fungal suspension was prepared by mixing some mold in 50 ml strile distilled water. The Orange sample used from the northern region of the country, Nowshahr, Iran, to extract the mold Penicillium (Fig.3.a). Then medium was prepared and cooled to room temperature. After cooling, some mold removed by strile loop from mildewy Orange sample on the plate containing medium to steep line (Fig.3.b) and then kept at room temperature for 4 days until growth is completed (Fig.3.c). After that it was kept in the refrigerator to be used for later testing.
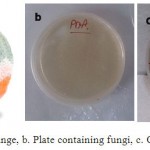 |
Figure 3: a. Mildewy Orange, b. Plate containing fungi, c. Completed growth of fungi |
After mold extraction and preparation of fungal suspension, suspension was rubed on the surface of the medium by strile swab. Then vesiculated in the center of the plate ( at middle of medium) by strile Pasteur pipette and 100 ml of various concentration of synthesized copper and copper oxide nanoparticles was poured by sampler into the eavity (Fig. 4). Results were evaluated after 48 h.
 |
Figure 4: Plates containing various concentration of synthesized copper and copper oxide nanoparticles
|
Results and discussion
The first step of the production of nanoparticles is changing the color from pale blue to yellow and then a brick or dark red that indicates the formation of Cu2O and turn it yellow to red represents that it is formation of copper nanoparticles. For copper oxide nanoparticles changing the color from blue to black color indicates the formation of copper oxide nanoparticles. These color changing are also observed (Fig. 5).
 |
Figure 5: a. Copper nanoparticles formation, b. Copper oxide nanoparticles formation
|
The second step of the synthesis and production of copper nanoparticles is using spectrophotometer and read the sulution absorbance at 550-620 nm wavelength range. Figure 6 exhibits the absorption spectrum of synthesized nanoparticles. Peak in this area represents the synthesis of copper nanoparticles. Copper nanoparticles was studied at 300-800 nm wavelength range and it has absorbance at 577 nm wavelength, so peak in this area represents the formation of copper nanoparticle. But the solution including copper oxide nanoparticles doesn’t represent any peak at 300-800 nm wavelength range. Because copper oxide nanoparticles has no peak at this wavelenght range.
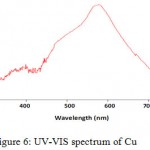 |
Figure 6: UV-VIS spectrum of Cu
|
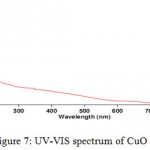 |
Figure 7: UV-VIS spectrum of CuO
|
X-ray diffraction analysis
The crystal structure of nanoparticles were verified by XRD analysis. Fig. 8 exhibits the XRD pattern of the as synthesized copper nanoparticles. Peaks observed at 2Ɵ values of 43.390, 50.490 and 74.180 correspond to (111), (200) and (220) planes of metallic Cu. These three peaks were quite consistent for the standard spectrum of the metallic Cu. Also, this analysis shows that the formation of particles are spherical. Diffraction peaks appeared at 36.540, 37.70, 53.40, 58.30, 620, 66.20 and 68.10 indicate the formation of copper oxide nanocrystals (Fig. 9).
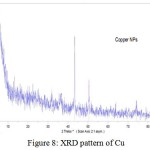 |
Figure 8: XRD pattern of Cu
|
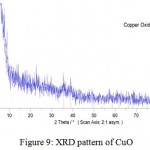 |
Figure 9: XRD pattern of CuO
|
Transmission electron microscopy analysis (TEM)
The morphology and size of the synthesized nanoparticles were examined by transmission electron microscopy. TEM analysis shows that the formation of particles are spherical and enjoy dispersal well (Fig. 10, 11). The XRD diffraction patterns were indicated with TEM analysis. The particle distribution graph of copper oxide nanoparticles were 7-8 nm size range. The particle size for copper nanoparticles were estimated 40 nm by device.
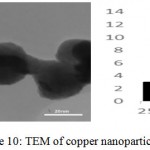 |
Figure 10: TEM of copper nanoparticles |
 |
Figure 11: TEM of copper oxide nanoparticles |
Anti-fungal properties results
As can be seen in Figure 12, fungal growth was numerous in control sample. But, in plates containing nanoparticles, zone of inhibition was seen that it proves the anti-fungal properties of synthesized nanoparticles in the present study. Zone of inhibition was measured by ruler. In the above figure can be seen that by increasing the concentration of nanoparticles to 15%, zone of inhibition was increased (Table 1).
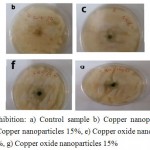 |
Figure 12: zone of inhibition: a) Control sample b) Copper nanoparticles 5%, c) Copper nanoparticles 10%, d) Copper nanoparticles 15%, e) Copper oxide nanoparticles 5%, f) Copper oxide nanoparticles 10%, g) Copper oxide nanoparticles 15%
|
Table 1: Anti-fungal activity of the nanoparticles on, fungus Penicillium, zone of inhibition (mm)
| zone of inhibition (mm) | Nanoparticles concentrate (%) | Nanoparticles type |
| 4 | 5 | Copper |
| 8 | 10 | Copper |
| 10 | 15 | Copper |
| 3 | 5 | Copper oxide |
| 6 | 10 | Copper oxide |
| 8 | 15 | Copper oxide |
Conclusion
Copper and copper oxide nanoparticles were synthesized by chemical reduction and precipitation methods. The crystal structure of nanoparticles were verified by XRD analysis. Peaks observed at 2Ɵ values of 43.390, 50.490 and 74.180 correspond to (111), (200) and (220) planes of metallic Cu. Also, diffraction peaks appeared at 36.540, 37.70, 53.40, 58.30, 620, 66.20 and 68.10 indicate the formation of copper oxide nanocrystals. The morphology and size of the synthesized nanoparticles were examined by transmission electron microscopy. TEM analysis shows that the formations of particles is spherical and enjoy dispersal well and also, the anti-fungal properties of synthesized nanoparticles were tasted against Penicillium on Orange fruit by Disc-Diffusion method. The antifungal properties of nanoparticles were studied by the effect of different concentrations of nanoparticles. The results show that anti-fungal properties also increased by increasing the concentration of nanoparticles to 15%.
Reference
- Ghorbani H. R. Biological and Non-Biological Methods for Fabrication of Copper Nanoparticles. Chem. Eng. J. 2015;202:1463.
CrossRef - Kotanarowshi A. Searching for possibilities of lubricating and cutting fluids modification with copper micro and nano particles. Mater. Sci. 2006;112:3.
- Khan, A., Rashid, A., Younas, R., Chong, R., A chemical reduction approach to the synthesis of copper nano particles. Int. Nano Lett. 2016;6:21.
CrossRef - Khanna P. K., Gaikwad S., Adhyapak P. V., Singh N., Marimuthu R. Synthesis and characterization of copper nano particles. Mater. Lett. 2007;61:4711.
CrossRef - Khodashenas B., Ghorbani H. R. Synthesis of copper nano particles : An overview of the various methods. Korean J. Chem. Eng. 2014;31:1105.
CrossRef - Yoon K. Y., Byeon J. H., Park J. H., Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nano particles. Int. Biodeterior. Biodegradation. 2007;373:572.
CrossRef - Usha R., Prabu E., Palaniswamy M., Venil C. K,,Rajendran K. R. Synthesis of metal oxide nanoparticles by Streptomyces Sp for development of antimicrobial textiles.Global J. Biotechnol. Biochem. 2010;5:153.
- Cuevas R., Durán N., Diez M. C., Tortella G. R., Rubilar O. Extracellular Biosynthesis of Copper and Copper Oxide Nanoparticles by Stereum hirsutum, a Native White-Rot Fungus from Chilean Forests. Jour. Nanomater. 2015;7.
CrossRef - Veerapandian M., Sadhasivam S., Choi J., Yun K. Glucosamine functionalized copper nanoparticles: Preparation, characterization and enhancement of antibacterial activity by ultraviolet irradiation.Chem. Eng. J. 2012;209:558.
CrossRef - Tamilvanan A., Balamurugan K., Ponappa K., Kumar B. M. Copper Nano particles Synthetic Strategies Properties and Multi functional Application. Int. J. Nanosci. 2014;13:1430001.
CrossRef - Wang Y., Cao G. Developments in Nano structured Cathode Materials for High-Performance Lithium-Ion Batteries. Adv. Mater. 2008;20:2251.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





