How to Cite | Publication History | PlumX Article Matrix
V. Krishnarjuna Reddy1, U. Keerthi1, A. V. N. Swamy2 and Dowlathabad Muralidhara Rao1
1Department of Biotechnology, Sri Krishnadevaraya University, Ananthapuramu, India.
2Department of Chemical Engineering, Jawaharlal Nehru Technological University Ananthapuram, Ananthapuramu, India.
Corresponding Author E-mail: rao.muralidhara@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2460
ABSTRACT:
The present paper deals with isolation of C.glutamicum strain and strain improvement of C.glutamicum by mutation studies for the production of L-Glutamic acid. Native isolate of C.glutamicumwas manipulated to excrete L-glutamate. The modified protocol was used in this paper to trigger L-glutamate excretion. The important feature of this protocol is to achieve a biotin concentration that is low enough to activate glutamate excretion but high adequate to still allow sufficient growth of the cells. To achieve this appropriate biotin concentration, a pre cultivation step is required in order to deplete the cells of biotin. Later Physical Mutation was induced on Biotin depleted C.glutamicum strain.
KEYWORDS:
Biotin;Corynebacterium glutamicum: Fermentation L-Glutamic acid;
Download this article as:| Copy the following to cite this article: Reddy C. V. K, Keerthi U, Swamy A. V. N, Rao D. M. Biotin Axotroph Mutants of Native Corynebacterium Glutamicum for the Fermentative Production of L-Glutamic Acid. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Reddy C. V. K, Keerthi U, Swamy A. V. N, Rao D. M. Biotin Axotroph Mutants of Native Corynebacterium Glutamicum for the Fermentative Production of L-Glutamic Acid. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22999 |
Introduction
Corynebacterium glutamicumwas originally used as bacterium forthe production of L-Glutamic acid.1 To achieve improved production of extracellular amino acid the changes in cellular metabolism are desired. L-Glutamic acid excretion by C. glutamicum is promoted by biotin limitation, treatment with penicillin, or by addition of fatty acid ester surfactants.2,3,4 Glutamate is often known as one of the primary products of nitrogen metabolism in the living cell and the glutamic dehydrogenase system correspond to an significant link between the metabolism of amino acids and carbohydrates. Glutamate is generally transformed rapidly to various other amino acids and proteinaceous compound.
Glutamic acid was first produced in Japanby hydrolysis of wheat, gluten or Soya bean protein in 1908. The fermentation process was invented by Kyowa Hakko Kogyo in 1957, while Ajinomoto Company produced Glutamic Acid synthetically5. Commercial Glutamic Acid is made by fermenting molasses from sugar beet or sugar cane, a process similar to making wine, beer, sauce, and vinegar.5 Higher levels of extracellular glutamate can induce seizures and excite toxic neuronal cell death. In glioma and other brain tumors, excessive glutamate free from tumor cells may be responsible for tumor associated necrosis and also possibly to capture caused due to peritumoral and tumoral brain tissue.6
Materials and Methods
Isolation of Corynebacterium Glutamicum
Isolation
Soil is a good source of microorganisms. Sewage contaminated soil samples were collected from local Anantapuramu sewage contaminated soil of Anantapuramu. The soil sample was serially diluted and plated on isolation medium and incubated for 48-72 hours at 30°C.7
A modified Bouillon medium was used for isolation of Corynebacterium glutamicum which has the following composition,8 (g/l) separately for each dilution. Peptone, 10.0, Meat extract, 5.0 Yeast extract, 2.0, Sodium chloride, 2.5 and Agar, 20.0.Ingredients were mixed in 1000ml distilled water and heated to dissolve completely. Cylohexamide at a concentration of 50mg/l was added to restrict the growth of fungi.9 The pH of the medium was adjusted to 7.0 by 0.1 N Sodium hydroxide,and sterilized by autoclaving for 15 minutes at 15 psi.
Pediococcus acidilactici NCIB-8018 (MTCC Customer Number 6650) which is a screening organism for glutamic acid producing bacteria was obtained from NCL, Pune.It was maintained on MRS Agar medium. Corynebacterium glutamicum ATCC 13032, a wild type culture was purchased from ATCC U.S.A. Corynebacterium glutamicum MH 20-22B microbial culture, a leucine auxotroph was procuredfrom ProfessorEggeling, Biotechnology Institute, Julich, Germany for academic purpose. Corynebacterium glutamicum ATCC 13032 and Corynebacterium glutamicumMH 20-22B, Were maintained on nutrient agar medium.
Serial Dilution
1gm of sewage contaminated soil sample was dissolved in 10 ml sterile distilled water and dilutions were carried out.An aliquot of each dilution was transferred to a petriplate containing growth media and speeded.Triplicates were maintained for each dilution and incubated for 72 hrs at 300C. Plates contained sufficient numbers (30-50) of discrete, well isolated colonies were selected as a source of culture to be evaluated for production of L-glutamic acid. The selected plates were termed as master plate.
Screening of Corynebacterium Glutamicum Strains
Medium of following composition was used for screening test,9 Glucose 25.0g/l, Yeastextract0.5g/l, Meat extract 0.5g/l, Ammonium sulphate7.0g/l, Di-potassium hydrogen phosphate7.0g/l,Potassium di-hydrogen phosphate 3.0g/l, Agar20.0g/l and salts Magnesium sulphate.7H2O 0.52mg/l, Ferrous sulphate.7H2O 0.014mg/l, Manganese sulphate .4 H2O 0.005mg/l, Sodium chloride0.010mg/l. 1.0 µg/l of Biotin used as a growth factor.
The screening method, which in essence represented a bioassay for glutamicacid, was carried out by replica plating bacterial isolates of various origins onto a series of plates containing screening medium. The replicated cultures were incubated for 72 hrs at 300C. After growth,the cells on the test plates were inactivated by a strong dose of UV radiation; Detection of L-glutamic acid produced on screening medium by individual colonies was done by “Bio-autographic technique”.9 The agar plates were overlayed with 10ml molten basal agar containing glutamate-auxotrophic bacterium, Pediococcus acidilactici NCIB-8018 (obtained from NCL, Pune). After incubation at 370C, the test plates were scored for appearance of a halo growth of the assay organism around some of the UV-killed colonies. The glutamic acid-producers were then recovered from a non- UV-irradiated nutrient medium master plate. .Pure cultures of positive colonies were developed by streak plate technique. These isolates were subjected to preliminary morphological, cultural, and physiological and biochemical tests.
Productionmedium
Production media contain ingredientssame as that of Screening medium without agar. 15 ml of production media was taken in 50 ml conical flasks and autoclaved at 15psi for 15 mins.
Cultivation of Selected Isolates
Each 15 ml production media containing flasks were inoculated with twoloop fulls of selected isolates from slant culture tubes and incubated for 72 hrs at 30°C in orbital shaker at 200 rpm.8
Identification and Characterization of Selected Isolates
Morphological Characteristics
The selected isolates were subjected to Gram staining,11 spore staining and flagellation.
Cultural Characteristics
Colonies obtained were allowed for culture tests such as growth, edge or surface structure, pigmentation.
Biochemicalcharacteristics
The selected isolates were subjected to biochemical tests such as Catalase test11, Carbohydrate fermentation test11, Methyl red test and Voges–Proskauer test,12 Indole test, Hydrogen sulphide test,12 Phosphatase test, Urease test,11 Litmus milk test, Nitrate reduction test,11 Casein hydrolysis test,12 Starch hydrolysis test test,11 Gelatin liquefaction test.
Molecular Characterization of Selected Isolates
Isolation of DNA from Suspected Isolates
16s rDNA studies conducted () to know the molecular characterization.
Biotin Auxotrophic Mutants Preparation
Biotin Auxotrophic mutants were prepared by following L Eggeling et al.13 Preculture was done on LB agar plates and CGXII minimal medium and CGXII minus biotin medium are prepared.
Glutamic Acid Assay
The assay contains in a total volume of 1 ml: 850 μl of buffer, pH 8.6 (75 mMtriethanolamine, 10 mM KH2PO4, 0.5% Triton X-100), 10 μl of 50 mM NAD+, 50 μlof 1.5 mM iodonitrotetrazolium chloride, 10 μl of diaphorase [350 U/ml]), 10 to100 μl of sample (max. 25 μM in assay), and 20 μl of glutamate dehydrogenase(1,000 U/ml). The reaction is acceptable to complete, it may take 20 min, and theabsorbance is read at 492 nm against a blank without glutamate. The extinctioncoefficient for the formazan is 19.9 mM cm–1. There are several commercial suppliersproviding kits for quantitative L-glutamate determination.
Mutagenesis
In the present study fermentative production of glutamic acid was carried out by both wild type and mutants of C.glutamicum. Mutagenesis was carried out by physical and chemical mutagens.In the processes of Physical mutagenesis actively growing culture of C. glutamicum was inoculated on fiveculture plates by spread plate technique and the plates were exposed to Ultra Violet light (irradiated) with a dose of300erg/mm2 for 30sec, 60sec, 90sec, 120sec and 150 sec respectively and incubated. The survivors of the mutagenesis were again subjected to a second dose of mutagenesis. Finally the survivors of second dose were usedin this study.
Qualitative and Quantitative Estimation of Glutamic Acid
The fermentation process was monitored for 6 days during which samples were taken every 24 hrs. Instantly aftersampling, pH of samples was measured and the centrifuged at 8000xg in order to separate the cells; supernatant was used for estimation.
Fermentation Media
The basal fermentation medium for glutamic acid production contained 36 g glucose, 1 g KH2PO” 1 g MgS04 • 7H20, 2 ppm each of Fe2+ (as FeSO,’ 7HP) and Mn2+ (as MnSO4H20), 100 I1g thiamine’ HCl, 0.24 g soybean hydrolysate (as total nitrogen), 4 ml of 0.1 % cresol red solution and appropriate amounts of biotin and urea per liter of deionized water. pH was adjusted to 7.0 by KOH before autoclaving.
Fermentation Studies of Corynebacterium Glutamicum
Two loops of Corynebacterium glutamicum cells were grown on nutrient agar strain maintenance plate for 24 hours at 30°C were inoculated into 20 ml of Rich medium (Seed broth) in a 250 ml conical flask and cultivated for 24 hours in orbital shaker at 30°C with 120 rpm. The Seed broth was transferred to 180 ml of Inoculum medium in an Erlenmeyer flask and was kept in orbital shaker at 30°C with 120 rpm for 40 hours. This Inoculum medium (Inoculum) was used to inoculate the Fermentation medium in the Bioreactor and batch fermentation was carried out for 72 hours.
Qualitative Analysis of L-Glutamic Acid
Thin layer chromatography was used for qualitative detection of glutamic acid. TLC pates of 0.2 mm thickness were prepared using adsorbent materialsilica gel. The fermented broth culture was centrifuged and supernatant was collected and the supernatant which served as source of glutamic acid. Supernatant spotted on TLC plates along with glutamic acid standard and developed in a solvent system containing a mixture of n-butanol, acetic acid and water (4:1:1:v/v) for about 6 hrs. After development the chromatogram was sprayed with 0.15% ninhydrin ethanol solution and was kept in oven at 110oC for 3 min8 spots of all amino acids were visualized. Rf value of the samples were compared with the Rf value of glutamic acid standard.The ninhydrin colour reaction14 was used for quantitative determination of L-glutamic acid where the absorbance was measured at 570 nm. 1ml of freshly prepared ninhydrin reagent was added to the equal volumes of supernatant and heated in water bath for 15 min. and cooled under running tap water. The absorbance was measured against blank without supernatant. The amount of glutamic acid present in the sample was estimated from the standard curve for L- glutamic acid.
Results and Discussion
Isolation and Identification Corynebacterium Glutamicum
Corynebacterium glutamicum strains were isolated from sewage contaminated soils of Anantapuramu. The modified Bouillon medium was used for isolation.8 Cyclohexamide 50mg/L was added in the medium9 to restrict the growth of fungi. Total 1500 bacterial isolates from sewage contaminated soils were isolated and tested for the L-glutamic acid production. The bacterial isolates producing L-glutamic acid were screened with the media containing carbohydrates, nitrogen source and supplementedwith inorganic salts.
Lederberg and Lederberg 1952 reported Replica plating technique was used to transfer the organisms from isolation medium to screening medium and Bioautographic technique using dehydrated L-glutamic acid assay medium and Pediococcus acidilactici NCIB -8018 as test organism. Following incubation at 370C for 24hrs the test organism grows in the immediate area surrounding any given colony that produced L-glutamic acid. Eighty one (81) isolates out of fifteen hundred (1500 ) were found to produce L-glutamic acid Eighty one isolates (81) showing positive results were subjected to purification in to the same medium to elevate their L-glutamic acid producing capability and to identify Corynebacterium glutamicum.
Inpreliminary morphological screening tests of isolates, Out of Eighty one (81) isolates only fourteen (14) isolates were observed to posses characteristic properties of Coryneform bacteria. These 14 suspected isolates of Coryneform bacterias were subjected toMorphological, cultural, bio-chemical, and physiological characteristics were recorded respectively .Out of 14 only 6 isolates were tentatively identified by taxonomic comparison of Corynebacterium glutamicum. These includes isolate no SKUDBT-6, SKUDBT-202, SKUDBT-490, SKUDBT-681, SKUDBT- 878, SKUDBT-1226.
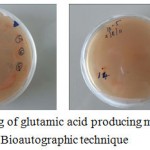 |
Figure 1: Screening of glutamic acid producing micro organisms by Bioautographic technique
|
The plates were layered with basal agar medium for glutamic acid bio-assay containing the assay organism Pediococcus acidilactici NCIB-8018 dispersed in the medium. Haloof growth developed around the colony producing glutamic acid during incubation at 370C. Colonies surrounded by halos on test plates were picked as glutamic acid producers.
 |
Figure 2: C.glutamicum AB763933 |
Molecular Characterization of Isolates
The Basic Protocol, using phenol extraction and ethanol precipitation, is appropriate for the purification of DNA from small volumes of suspected bacterial samples. Equal volumes of isopropanol and DNA solution are used in precipitation. Note that the isopropanol volume is half that of the given volume of ethanol in precipitations. This allows precipitation from a large starting volume in a single micro centrifuge tube. Isopropanol is less volatile than ethanol and takes longer to remove by evaporation. Some salts are less soluble in isopropanol (compared to ethanol) and will be precipitated along with nucleic acids. Extra washings may be necessary to eliminate these contaminating salts.
16s rRNA gene was amplified from purified genomic DNA with
16sF(5´AGAGTTTGATCCTGGCTCAG3´)and16sR(5´GGTTACCTTGTTACGACTT-3´) primers. The amplified gene was sequenced by using Big Dye chemistry soft ware. A260/280 ratio of the above DNA was recorded and the purity of the DNA was good.
Based on 16s rDNA partial sequence analysis with ChromasPro, Sequencing Analysis software the six strains showing similar characteristics of C. glutamicum and were the new variants of C. glutamicum and showing 92% similarity with C. glutamicum K051 strain (ATCC sub-strain K051).The 16s rDNA partial sequence was submitted to NCBI and got the Accession Number AB763933. 16s rDNA partial sequencing analysis of the remaining eight strains clears that they are new variants of Bacillus flexus,Bacillus thuringiensis,Bacillus stratosphericus,the sequences were submitted to NCBI with Accession Numbers AB686280,AB686281,AB701758.
Conclusion
The native isolate with accession AB763933 was converted into biotin auxotroph mutant. The native biotin auxotrophic mutantexposed to U.V mutation. Resultant U.V mutant was studied for its fermentation efficiency by using standard glutamic acid production media13. The strain yields 15.5 g/L, which is very slight increase of production with C. glutamicum improved strains. Further intensive molecular based strain improvement studies may be necessary to improve the L-Glutamic acid yield by using suitable fermentation medium and easy downstream process.
Acknowledgements
We would like to thanks Dr. DMR, Sri Krishnadevaraya University and JawaharlalNehru University Anathapuram for their support in this research.
Conflict of Interest
There is no conflict of interest
References
- Hwang J. H., Hwang G. H., Cho J. Y. Effect of increased glutamate availability on L-ornithine production in Corynebacterium glutamicum .J. Microbiol. Biotechnol. 2008;18:704-710.
- Hirasawa T., Wachi M.,Nagai K. L-Glutamic production by lysozyme-sensitive Corynebacterium glutamicum A mutant strains. BMC Biotehnolog. 2001;(1):9-13.
- Choi S, Nihira T., Yoshida T. Enhanced glutamic acid production of Brevibacterium sps with temperature shiftup cultivation. Bioscience Bioeng. 2004;(98)211-213.
- Amin G. A., AL-Talhi A. Production of L-Glutamate acid by immobilized cell reactor of the bacterium Corynebacterium glutamicumentrapped into carageenan gel bead. World Appl. Sci. 2007;(2):62-67.
- Ikeda K. A production method of seasoning mainly consists of salt of L-glutamic acid. Japanese patent. 1908.
- Shaw P. J. Motor newron disease. Br Med J. 1993;318:1118-21.
CrossRef - Keerthi U., Mokula M. d. Rafi., Gowramma B., Razak A. M., Reddy C. V. K., Swamy A.V. N.,Rao M. D. Fermentative Production of L-Glutamatic Acid by the Bacillus Species Isolated from Sewage Contaminated Soil. European Journal of Pharmaceutical and medical Research. 2016;3(2):214-218.
- Kinoshita S., Udaka S., Shimono M. Studies on the amino acid fermentation. Part 1:- Production of L-Glutamic acid by various microorganisms. J.Gen. Appl. Microbial. 1957;(3):193-205.
- Daoust D. R. Microbial synthesis of amino acids. In: Industrial Microbiology. Miller, B.M. and Litsky, W. McGraw-Hill Book Company, New York. 1976;106–127.
- Cappuccino J. G. N. Sherman Microbiology – a Laboratory Manual. 1996;137–49.
- Benson H. J. Microbiological Applications Laboratory Manual in General Microbiology. 8th Ed. McGraw-Hill, New York, NY. 2002;58-60.
- Seeley H.W., Van Demark P. J. Microbes in Action: A Laboratory Manual of Microbiology, 3rd Ed, W.H. Freeman and Company, USA. 1981.
- Handbook of Corynebacterium glutamicum By Lothar Eggeling, Michael Bott. 2005.
- Meyer H. The Ninhydrin Reaction and Its Analytical Applications. 1957.

This work is licensed under a Creative Commons Attribution 4.0 International License.





