How to Cite | Publication History | PlumX Article Matrix
Mayson H. Alkhatib, Dunya A. Nori and Maryam A. Al-Ghamdi
Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
Corresponding Author E-mail: mhalkhatib@kau.edu.sa
DOI : http://dx.doi.org/10.13005/bbra/2419
ABSTRACT: Sorafenib (SOR), a chemotherapeutic agent, is a multikinase inhibitor that has a cardiotoxic and hematoxic effect which could limit its application in cancer therapy. In the current study, SOR was incorporated into a nanoemulsion (NE) containing flaxseed oil (SOR-NE) and its antitumor effect was examined in Ehrlich ascites carcinoma (EAC)-bearing female Swiss Albino mice. Fifty mice were divided into five groups (n =10). Groups I and II served as untreated mice and untreated EAC-bearing mice, respectively, while groups III-V represented the EAC-mice administered orally with blank NE, SOR dissolved in Cremophor (SOR-Chremo) and SOR- NE, respectively. It has been found that SOR-NE group has lesser counts of white blood cells and decreased levels of cardiac enzymes and relatively normal heart tissue when compared to the SOR-Chremo group. Encapsulating SOR in NE has decreased its cytotoxicity on the heart and blood.
KEYWORDS: Chemotherapeutic agents; Cytotoxicity; Cardiac enzymes Flaxseed oil; Nanoparticle;
Download this article as:| Copy the following to cite this article: Alkhatib M. H, Nori D. A, Al-Ghamdi M. A. Cardiotoxicity and Hematoxicity of Sorafenib – Loaded Nanoemulsion in Ehrlich Ascites Carcinoma - Bearing Mice. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Alkhatib M. H, Nori D. A, Al-Ghamdi M. A. Cardiotoxicity and Hematoxicity of Sorafenib – Loaded Nanoemulsion in Ehrlich Ascites Carcinoma - Bearing Mice. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22119 |
Introduction
Cancer is one of the most common diseases that is raising mortality worldwide.1 Cancer is defined as the abnormal growth of cells that disturb the cellular structures and function, leading to stimulation of the production of blood vessels in order to maintain the tumor cell proliferation through supplying them with oxygen and nutrients.2 In spite of the extensive researches performed on cancer therapy, pharmaceutical industries are still facing challenges in formulating the chemotherapeutic agents which have side effects that could adverse cancer treatment.3
Sorafenib (BAY 43-9006, Nexavar, SOR) is a novel bis-aryl urea compound. It has anti-proliferative and anti-angiogenic properties because it is a multikinase inhibitor of rapidly accelerated fibrosarcoma (RAF) kinase and receptor tyrosine kinases of multiple proangiogenic factors such as vascular endothelial growth factor receptor, platelet-derived growth factor receptor and c-Kit (Mast/stem cell growth factor receptor).4 SOR has been demonstrated to have anticancer activity in colon, breast, non–small-cell lung cancer xenograft models and human leukemia cells.5 Additionally, SOR combined with chemotherapeutic agents, gemcitabine and capecitabine, was found to have a great effect in treating patients with HER2-negative advanced breast cancer.6 Due to the pleiotropic effect of SOR as multi targeted kinase inhibitors, SOR significantly ameliorated the survival of the patients with iodine-refractory differentiated thyroid cancer.7
Although SOR has antitumor and antimalignancy effect, it has a major drawback. In fact, the antigiogenesis property of SOR could impair the blood vessel formation of both of the cancer and healthy cells, especially, the myocytes. The death of myocytes could cause myocardial infarction and arterial hypertension.8 There have been many attempts to reduce the cardiotoxicity of SOR by delivering it in nanocarriers. It has been found that SOR, loaded in solid lipid nanoparticles consisting of tripalmitin, Captex 355 EP/NF and Miglyol 812, has greater anticancer effect than the free drug when subjected into the hepatocellular carcinoma cell lines.9 In addition, Zhang et al.10 demonstrated that encapsulating SOR with the iron oxide nanoparticle in polymeric micelles has stimulated apoptosis in hepatic cancer cells in vitro. Moreover, incorporating SOR into albumin nanoparticles containing paclitaxel has lowered the haemolysis and myelosuppression effect of both drugs and raised their antitumor effect.11
Nanoemulsion (NE) based on essential oils would be promising nanocarrier for the chemotherapeutic agents. NEs are suspension systems that consist of water, oil, surfactants and cosurfactants. They contain nanodroplets with diameter sizes in the range of 20 – 200 nm12. The essential flaxseed oil, consists mainly of α-linolenic acid (ALA) is naturally rich in antioxidants like tocopherols and beta- carotene. Flaxseed oil has potential health benefits as it has anticancer activity and has the potential to reduce dyslipidemia, inflammation and cardiovascular diseases.13 The objective of the current study was to evaluate the hematoxicity and cardiotoxicity of SOR when loaded in NE-based-flaxseed oil in the EAC-bearing female Swiss Albino mice.
Materials and Methods
Materials
Flaxseed oil was obtained from Sokar nabat for natural oils (Jeddah, KSA). Polyoxyethylene-20-sorbitan monooleate (Tween 80), sorbitan laurate (span 20) and distilled water were purchased from Al-rowad modern establishment for the supply of medical equipment (Jeddah, KSA). SOR as a tosylate Bayer Company product (Nexavar film coated tablets) was obtained from Al-Foad Pharmacy (Cairo, Egypt). The Cremophor® RH 40, Ethyl Alcohol 95%, phosphate buffered saline (PBS) were purchased from Sigma- Aldrich (Missouri, USA). Serum analysis kits were obtained from Human Biochemical and Diagnostic (Wiesbaden, Germany) and Crescent diagnostics Company, Saudi Arabia. Commercial pelleted mice food, which contains 20% protein, 4% fat, 3.5% fiber, 6% ash, 0.5% salts, 1% calcium, 0.6% phosphor, vitamin less than 80 IU/kg and 2850 kcal/ kg of Energy, was obtained from Saudi Grains Organization (Jeddah, KSA).
Animals and Subjects
Fifty female Swiss Albino mice (mean weight 24-30 g) were housed in large cages with five mice per cage. The animals were adapted under standard laboratory conditions (temperature 25°±2°C with dark/light cycle 12/12 h) for 2 days before the initiation of the experiments. One hundred gram of pelleted food for each cage per day and tap water were given through the experimental period ad libitum. The animals were maintained in accordance with King Abdulaziz University’s policy and the International Ethical Guidelines on the care and use of laboratory animals.14 The ethical approval was obtained from the research ethics committee in the Faculty of Medicine at King Abdulaziz University. EAC cells were procured from American Type Tissue Culture Collection (Manassas, VA, USA).
Preparation of NE formulations
The blank NE formulation (Blank NE) was prepared by mixing 15% (v/v) of surfactant mixtures of span 20 and Tween 80 at a ratio of 1:1.5, respectively, 61% (v/v) of flaxseed oil and 24% (v/v) of distilled water. Then, the mixture was vortexed with continuous heating in a water bath for more than a month at 120 °C. The mixture has completely got converted from turbidity to clear and transparent solution as shown in Figure 1. The SOR-loaded-NE (SOR-NE) was prepared by dissolving directly a 30 mg of SOR/ kg of mouse body weight in 0.1 ml of Blank NE. The SOR-Chremo, prepared as described by Wilhelm et al.15 and Kawazoe et al.,16 was made by dissolving a 30 mg of SOR/ kg of mouse weight in 0.1 ml of stock solutions of Cremophor and 95% Ethyl Alcohol (Chremo) at an affixed ratio of 1:1.
 |
Figure 1: The process of producing the NE at different period of time. From left to right, time has increased from the 0th day to 7th day to 14 th days.
|
Characterization of the Prepared NE formulation
The particle size analysis and the morphologies of the NE droplets were determined using a Scanning Electron Microscope (SEM) (Quanta FEG 250, Holland) at the National Research Center, Cairo, Egypt. The samples were dehydrated, affixed and placed in a gold coater. Following the completion of gold coating, samples were viewed through the SEM.
In Vivo Antitumor Activity of Drug formulations
Transplantation of EAC Cell Line in the Mice
The transplantation of the EAC cells in the mice was implemented according to a method adopted by Alkreathy et al.17 The EAC cells were preserved in the ascitic form by consecutive passages in mice every 10 days. Ascitic fluid was withdrawn from EAC bearing mice on the 8th day following transplantation. Before the tumor inoculation, the viable EAC cells, assembled from the peritoneal cavity, were counted by Trypan Blue exclusion test.
The mice were divided into five groups at which each group has 10 mice. The body weight of all mice was taken before injection with EAC cells. All of the mice in each group, except group I, were injected i.p. with 2.5×106 EAC cells/mouse for forty eight-hour incubation. Group I served as the negative control (untreated mice) while group II was the control positive (EAC+). Groups III-V were treated with the blank NE, SOR-Chremo and SOR-NE, respectively, at a dose of 30 mg/kg of mouse every other day for a total of 7 doses via oral gavage administration.8
On the 16th day, mice from each group were set aside fasting for 12h and the body weight of each mouse was taken on a digital scale. Then, the blood was collected by a retro orbital plexus method for the measurements of hematological and biochemical parameters. Finally, these animals were sacrificed in order to collect their organs for the histological study.
Organ weight
Immediately following sacrifice, the hearts of all of the slaughtered mice were removed, washed with normal saline solution, dried on blotting paper and weighed on a digital scale. To compute the organ weight –to– body weight ratio, the post-sacrifice organ weight (B) will be divided by the pre-sacrifice body weight of the same animal (A).
Hematological and Serum Biochemical Assays
The collected blood was exploited to measure the white blood cells (WBCs), red blood cells (RBCs), hemoglobin (Hb) and platelets by using complete blood count (CBC) analyzers, purchased from Beckman Coulter, California, U.S.
For Biochemical assays, the blood samples were allowed to clot and the serum was separated by centrifugation at 3000 RPM for 15 min. Serum was utilized for the detection of heart function. Lactate dehydrogenase (LDH), creatine phosphokinase (CK), and creatine phosphokinase-MB (CK-MB) were determined by the optimized standard method using a commercial kit purchased from the German Society for Clinical Chemistry (Germany) through Human Company. Additionally, the lipid profile, included cholesterol (CHO), high density lipoprotein (HDL) and triglyceride (TG), was detected by the optimized UV-test according to the International Federation of Clinical Chemistry (IFCC) (Crescent diagnostics Company, Saudi Arabia).
Histology
The collected heart tissues were fixed at 10% neutral buffered formalin (4% formaldehyde in phosphate buffered saline). Afterwards, the tissues were incrementally dehydrated by decreasing concentrations of alcohol and then stained with hematoxylin and eosin (H & E), followed by microscopic examination.
Statistical Analysis
The statistical assessments were employed to determine the significant differences between the samples through using one-factor analysis of variance (ANOVA) and the t-test, implemented by MegaStat Excel (version 10.3, Butler University). The considerable variations between the samples were identified at p-value <0.05.
Results
Sem images
Figure 2 reveals SEM images of Blank NE and SOR-NE, respectively. The average size diameters of Blank-NE droplets were 105.98 ± 23.05 nm with PDI of 0.22 whereas the average size diameters of SOR-NE has decreased to 29.79 ± 3.71 nm with PDI of 0.12. The nanodroplets of both formulas were spherical.
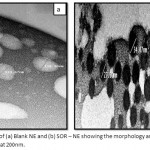 |
Figure 2: SEM images of (a) Blank NE and (b) SOR – NE showing the morphology and size measurements. Images are magnified at 200nm.
|
Hematological Parameters
All tested hematological parameters of experimental groups are summarized in Table 1. WBCs counts have significantly got enhanced in the treated groups compared to the normal group. Interestingly, both of Blank NE and SOR-NE groups have less increment in WBCs counts, which were slightly elevated from the standard range, than EAC+ and SOR-Chremo groups. In terms of RBCs counts, both of SOR-Chremo and SOR-NE groups have the least counts among all of the groups. Nevertheless, all of the experimental groups have RBCs counts within the standard range.
Table 1: The effect of drug formulations on the hematological parameters of the experimental groups. Data were expressed as
![]()
| Groups | Total WBCs
(5-12 × 103) cell/µl |
Total RBCs
(7-13 × 106) cell/µl |
Hb
g/dl |
Platelets
(×103 cells/µl) |
| Normal | 7.2 ± 2.30 b | 10.5 ± 2.10 | 12.56 ± 0.83 | 840 ± 95.65 |
| EAC+ | 17.04 ± 5.11 a | 9.65 ± 2.03 | 14.17 ± 2.41 | 998.57 ± 202.13 |
| Blank NE | 13.3 ± 3.4 ad | 9.1 ± 2.0 | 12.73 ± 0.80d | 1030.5 ± 200.17d |
| SOR – Chremo | 22.41 ± 6.39ab | 8.13 ± 0.77a | 15.96 ± 0.90a | 707.71 ± 124.86b |
| SOR – NE | 12.74 ± 2.63ac | 8.48 ± 1.19a | 15.49 ± 3.05a | 699.71 ± 273.9b |
a There is a significant difference between the desired group and the normal group; b There is a significant difference between the desired group and the EAC + group; c There is a significant difference between the SOR – Chremo and SOR – NE groups; d There is a significant difference between the SOR – Chremo and the Blank NE groups.
Furthermore, the amounts of Hb have significantly increased in SOR-Chremo and SOR-NE when compared to the normal group. In fact, the amounts of Hb in EAC+ and Blank NE were comparable to the normal group. Regarding the platelets counts, the treated groups have equivalent counts with the normal group. Among the treated groups, the platelet counts of both of SOR-Chremo and SOR-NE groups have the least counts.
Heart function
Heart-to- Body weight Ratio
Table 2 displays the effect of the drug formulations on the heart- to- body weight ratio. The relative heart weights of all treated and EAC+ groups are significantly less than the normal group. In addition, a considerable enhancement in the relative heart weight of SOR-NE group was observed compared to SOR-Chremo group.
Table 2: The Effect of the drug formulations on the heart -to-body weight ratio of the experimental groups. Data were expressed as ± SD.
| Groups | Heart –to- body weight ratio | LDH
(120 -240 U/L) |
CK
(10-70 U/l) |
CK- MB (up to 10 U/l) |
| Normal | 0.0051± 0.0009b | 133 ± 2.6 | 32.25 ± 3.44 | 5.5 ± 0.28b |
| EAC+ | 0.0028 ± 0.0004a | 143.67 ± 11.59 | 26.83 ± 6.19 | 0.64 ± 0.42 |
| Blank NE | 0.0033 ± 0.0004a | 130.33 ± 14.15d | 36.45 ± 13.12d | 5.45 ± 1.39bd |
| SOR- Chremo | 0.0037 ± 0.0006ab | 218.33 ± 5.78ab | 57.77 ± 5.46ab | 13.2 ± 0.73ab |
| SOR- NE | 0.0043 ± 0.0007abc | 151.67 ± 20.43c | 39.44 ± 9.25c | 9.08 ± 1.64abc |
a There is a significant difference between the desired group and the normal group; b There is a significant difference between the desired group and the EAC + group; c There is a significant difference between the SOR – Chremo and SOR – NE groups; d There is a significant difference between the SOR – Chremo and the Blank NE groups.
Serum analysis
The effect of drug formulations on the LDH, CK and CK-MB enzymes of the experimental groups are illustrated in Table 2. In terms of LDH levels, the Blank NE and SOR–NE groups did not considerably differ from the normal and EAC+ groups. In contrast, the SOR- Chremo group has a significant increase in LDH level relative to all of the groups.
Furthermore, the levels of the CK enzyme in all of the experimental groups were within the standard range. In fact, the CK levels of SOR- NE group has decreased when compared to the SOR-Chremo group. Regarding the CK-MB levels, all of the groups have levels within the standard range except SOR-Chremo group has elevated level. Interestingly, mice treated with SOR-NE has CK-MB levels that are significantly lesser than SOR-Chemo group. Nevertheless, the drug treated groups, SOR-NE and SOR Chremo, have CK-MB levels that are significantly greater than all of the other groups.
Heart histology
Figures 3 – 5 exhibit the histopathological examination of the heart tissues of the experimental groups. Figure 3A shows a normal structure of the cardiac myocytes with their centrally placed nuclei of the normal group. Figure 3B has revealed the heart tissue of the EAC+ group which has cardiac myocytes structure comparable to the normal group with dilated blood vessels and mild changes in few nuclei in the form pyknosis, which is a condensation of chromatin in the nucleus of a cell undergoing necrosis or apoptosis, and karyolysis, which is known as the dissolution of a cell nucleus. The tissue section of the heart of the Blank NE treated group presented similar tissue structure to the EAC+ group as exhibited in Figure 4. The heart microscopic tissues of the mice treated with SOR-Chremo, shown in Figure 5A, displayed noticeable damage of cardiac myocytes with dilated blood vessels and excessive changes in the nucleus in the form of pyknosis and karyolysis. In contrast, the heart tissue section of mice treated with SOR-NE showed less dilated blood vessels with less presence of the pyknosis and karyolysis in the nuclei as exhibited in Figure 5B.
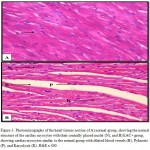 |
Figure 3: Photomicrographs of the heart tissues section of A) normal group, showing the normal structure of the cardiac myocytes with their centrally placed nuclei (N), and B) EAC+ group, showing cardiac myocytes similar to the normal group with dilated blood vessels (B), Pyknosis (P), and Karyolysis (K). H&E x 400
|
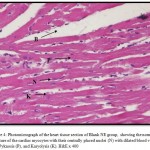 |
Figure 4: Photomicrograph of the heart tissue section of Blank NE group, showing the normal structure of the cardiac myocytes with their centrally placed nuclei (N) with dilated blood vessels (B), Pyknosis (P), and Karyolysis (K). H&E x 400
|
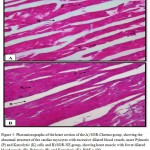 |
Figure 5: Photomicrographs of the heart section of the A) SOR-Chremo group, showing the abnormal structure of the cardiac myocytes with excessive dilated blood vessels, more Pyknotic (P) and Karyolytic (K) cells and B) SOR-NE group, showing heart muscle with fewer dilated blood vessels (B), Pyknosis (P), and Karyolysis (K). H&E x 400
|
Lipid Profile
Table 3 illustrates the effect of the drug formulations on the lipid profile of the experimental groups. The amounts of the CHO in all of the treated groups showed no significant change when compared to the normal and EAC+ groups. The variations in HDL levels of the experimental groups were noticeable. SOR-NE and SOR-Chremo groups have HDL levels comparable to the normal group and significantly greater than the EAC+ group. In terms of the TG levels, all of the experimental groups have levels within the standard range. In fact, SOR – NE group have the largest TG levels when compared to the normal and EAC+ groups.
Table 3: Serum analysis of the experimental mice groups treated with different drug formulations in order to detect the lipid profile. Data were expressed as.
| Groups | CHO
(5.1-6.2mmol/l)
|
HDL
(>1.68mmol/l) |
TG (0 .4-1.86 mmol/l) |
| Normal | 4.8 ± 1.23 | 2.8 ± 0.54b | 0.7 ± 0.24 |
| EAC+ | 4.38 ± 2.21 | 1.45 ± 0.20a | 0.76 ± 0.11 |
| Blank NE | 3.6 ± 0.99 | 2.09 ± 0.37 | 0.83 ± 0.41 |
| SOR- Chremo | 6.13 ± 1.15 | 2.94 ± 0.63b | 0.80 ± 0.26 |
| SOR- NE | 4.68 ± 2.27 | 3.27 ± 1.05b | 1.38 ± 0.51ab |
a There is a significant difference between the desired group and the normal group; b There is a significant difference between the desired group and the EAC + group; c There is a significant difference between the SOR – Chremo and SOR – NE groups; d There is a significant difference between the SOR – Chremo and the Blank NE groups.
Discussion
SOR is considered a potent anticancer agent. However, it has a challenge in being used for cancer treatment because of the complications it causes for the cardiovascular system.8,18 SOR causes mainly hypertension since it induces necrosis in the myocytes which could result in cardiac ischemia. In the present study, SOR was incorporated into NE containing flaxseed oil to reduce cardiotoxicity. Our data showed that the reduction in the relative heart weight of the EAC+ and treated groups, compared to the normal group, can be explained by the body weight enhancement resulted from the inoculated tumor. In other words, there is an inverse relationship between the relative heart weight and the body weight. Interestingly, SOR-NE group has the greatest relative heart weight among the EAC+ and treated groups which could be due to the reduction in the tumor weight. Consistent with our results, SOR-treated hearts had considerably smaller heart weights.8
Furthermore, the elevated levels of LDH, CK-MB and CK of the SOR-Chremo group have noticeably decreased in the SOR-NE group to be within the standard ranges. In fact, the enzyme levels did not significantly change in the Blank NE treated group. In addition, the cardiac myocytes of the heart tissue of the SOR-Chremo group were clearly damaged while the tissues of the SOR-NE and Blank NE were similar to the normal group. A clinical study has demonstrated that most of the patients who were administered with SOR have had elevated levels of CK, CK-MB and troponin T although they were not having a history of heart disease.19
In addition to the SOR cardiotoxicity, SOR was found to be hematoxic as it raised Hb, WBC and platelet levels in the blood in patients subjected to SOR for 21 days.20 Another recent study had demonstrated that administering SOR with olmesartan, a hypertension medication, into EAC-bearing mice had disturbed the components of the blood. 21 The current study showed that loading SOR in NE based-flaxseed oil has reduced its effect on the WBCs. The presence of Flaxseed oil in the drug formula might reduce the inflammatory effect of SOR since flaxseed oil contain ALA, which has the potential to impede Interleukin 6, Interleukin 1β, and tumor necrosis factor-α production by peripheral blood mononuclear cells.22 Additionally, the reduction in the inflammatory cytokines caused by ALA could benefit the flaxseed oil in having a cardioprotective effect.
In the present study, encapsulating SOR in NE has decreased the droplet diameter of NE from 105.98±23.05 to 29.79±3.71 suggesting that SOR was actually loaded in the NE. There was a normal distribution of SOR-NE droplets as the PDI (0.12) was less than 0.25. A study implemented by Kyoto et al.23 has demonstrated that the small droplet size of NE loaded with drugs would improve the absorption of drug in the intestinal tract. In 2011, Souto and his collaborators24 have reported that NEs loaded with hydrophobic drugs have greater oral bioavailability in the systemic circulation.
Conclusion
It has been found that loading SOR in NE –based-flaxseed oil has decreased the cardiotoxicity and hematoxicity of SOR. NE was prepared by mixing flaxseed oil with span 20, Tween 80, and distilled water. The mice treated with blank NE showed no signs of toxicity of the NE on the heart and blood as estimated through the biochemical analysis which indicate a safe effect of NE. In conclusion, the NE-based- flaxseed oil was a good candidate as a nanocarrier for SOR.
Acknowledgements
We sincerely thank King Abdulaziz City for Science and Technology for its financial support to the research project designated by a number (PGP-38-11).
Conflict of Interest
The authors declare no conflict of interest associated with this work.
References
- Torre L. A., Sauer A. M. G., Chen Jr M. S., Kagawa-singer M., Jemal A and Siegel R. L. Cancer Statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging Incidence in Males and Females. CA Cancer J Clin. 2016;66(3):182–202. http://doi.org/10.3322/caac.21335.
CrossRef - Eichholz A., Merchant S and Gaya A. M. Anti-angiogenesis therapies: Their potential in cancer management. Onco Targets and Therapy. 2010;24(3):69–82. http://doi.org/10.2147/OTT.S5256.
CrossRef - Nussbaumer S., Bonnabry P., Veuthey J. L and Fleury-Souverain S. Analysis of anticancer drugs: A review. Talanta, 2011;85(5):2265–2289. http://doi.org/10.1016/j.talanta. 2011.08.034.
CrossRef - Wilhelm S., Carter C., Lynch M., Lowinger T., Dumas J., Smith R. a., Kelley S., Schwartz B and Simantov R. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nature Reviews. Drug Discovery. 2006;5(10):835–844. http://doi.org/10.1038/nrd2130.
CrossRef - Rahmani M., Davis E. M., Bauer C., Dent P and and Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. Journal of Biological Chemistry. 2005;280(42):35217–35227. http://doi.org/10.1074/jbc.M506551200.
CrossRef - Gradishar W. Sorafenib in locally advanced or metastatic breast cancer. Expert Opinion on Investigational Drugs. 2012;21(8):1177–1191. http://doi.org/10.1517/13543784.2012.689824.
CrossRef - Park C., Perini J., Farmer R. W., Fancy T., Monga M and Remick S. C. Sorafenib and thyroid cancer. BioDrugs. 2013;27(6):615–628. http://doi.org/10.1007/s40259-013-0049-y.
CrossRef - Duran J. M., Makarewich C. A., Trappanese D., Gross P., Husain S., Dunn J., Lal H., Sharp T.
E., Starosta T., Vagnozzi R. J., Berretta R. M., Barbe M., Yu D., Gao E., Kubo H., Force T and Houser S. R. Sorafenib cardiotoxicity increases mortality after myocardial infarction. Circulation Research. 2014;114(11):1700–1712. http://doi.org/10.1161/CIRCRESAHA.114.303200.
CrossRef - Bondì M. L., Botto C., Amore E., Emma M. R., Augello G., Craparo E. F and Cervello M. Lipid nanocarriers containing sorafenib inhibit colonies formation in human hepatocarcinoma cells. International Journal of Pharmaceutics. 2015;493(1–2):75–85. http://doi.org/10.1016/j.ijpharm.2015.07.055.
CrossRef - Zhang L., Gong F., Zhang F., Ma J., Zhang P and Shen J. Targeted therapy for human hepatic carcinoma cells using folate-functionalized polymeric micelles loaded with superparamagnetic iron oxide and sorafenib in vitro. International Journal of Nanomedicine. 2013;8:1517–1524. http://doi.org/10.2147/IJN.S43263.
CrossRef - Zhang J. Y., He B., Qu W., Cui Z., Wang Y., Zhang H., Wang C., Zhang Q. Preparation of the albumin nanoparticle system loaded with both paclitaxel and sorafenib and its evaluation in vitro and in vivo. Journal of Microencapsulation. 2011;28(6):528–536. http://doi.org/10.3109/02652048.2011.590614.
CrossRef - Jaiswal M., Dudhe R., Sharma P. K. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–127. http://doi.org/10.1007/s13205-014-0214-0.
- Goyal, A., Sharma, V., Upadhyay, N., Gill, S., Sihag, M. Flax and flaxseed oil: an ancient medicine & modern functional food. Journal of Food Science and Technology, 2014; 51(9): 1633–1653. http://doi.org/10.1007/s13197-013-1247-9.
CrossRef - National Research Council (US) Committee for the Update of the Guide for the Care and Use of LaboratoryAnimals. Guide for the Care and Use of Laboratory Animals. Eight edn. Washington, D.C. : National Academies Press. 2011.
- Wilhelm S. M., Carter C., Tang L., Wilkie D., McNabola A., Rong H., Chen C., Zhang X., Vincent P., McHugh M., Cao Y., Shujath J., Gawlak S., Eveleigh D., Rowley B., Liu L., Adnane L., Lynch M., Auclair D., Taylor I., Gedrich R., Voznesensky A., Riedl B., Post L. E., Bollag G and Trail P. A. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF / MEK / ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the Pr. Cancer Res. 2004;64(19):7099–7109. http://doi.org/10.1158/0008-5472.CAN-04-1443.
CrossRef - Kawazoe H., Bilim V. N., Ugolkov A. V., Yuuki K., Naito S., Nagaoka A., Kato T and Tomita Y. GSK-3 inhibition in vitro and in vivo enhances antitumor effect of sorafenib in renal cell carcinoma (RCC). Biochemical and Biophysical Research Communications. 2012;423(3):490–495. http://doi.org/10.1016/j.bbrc.2012.05.147.
CrossRef - Alkreathy H. M., Damanhouri Z. a., Ahmed N., Slevin M and Osman M. M. Mechanisms of Cardioprotective Effect of Aged Garlic Extract Against Doxorubicin-Induced Cardiotoxicity. Integrative Cancer Therapies. 2012;11(4):364–370. http://doi.org/10.1177/1534735411426726.
CrossRef - Abdel-Qadir H., Ethier J. L., Lee D. S., Thavendiranathan P and Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treatment Reviews. 2017;53:120–127. http://doi.org/10.1016/j.ctrv.2016.12.002.
CrossRef - Schmidinger M., Zielinski C. C., Vogl U. M., Bojic A., Bojic M., Schukro C., Ruhsam M., Hejna M and Schmidinger, H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology. 2008;26(32):5204–5212. http://doi.org/10.1200/JCO.2007.15.6331.
CrossRef - Awada A., Hendlisz A., Gil T., Bartholomeus S., Mano M., Valeriola D. de Strumberg D., Brendel., Haase C. G., Schwartz B and Piccart M. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 21 days on/7 days off in patients with advanced, refractory solid tumors. British Journal of Cancer. 2005;92(10):1855–1861. http://doi.org/10.1093/annonc/mdi310.
CrossRef - Abd-alhaseeb M. M., Zaitone S. A., Abou-el-ela S. H and Moustafa Y. M. Assessment of the Safety of Olmesartan in Combination with Sorafenib in Mice Bearing Ehrlich ’ s Ascites Carcinoma. Journal of Cancer Therapy. 2013;4:1355–1361.
CrossRef - Zhao G., Etherton T. D., Martin K. R., Gillies P. J., West S. G., Kris-Etherton P. M. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. American Journal of Clinical Nutrition. 2007;85(1):385–391. http://doi.org/85/2/385 .
- Kotta S., Khan A. W., Pramod K., Ansari S. H., Sharma R. K and Ali J. Exploring oral nanoemulsions for bioavailability enhancement of poorly water-soluble drugs. Expert Opinion on Drug Delivery. 2012;9(5):585–598. http://doi.org/10.1517/17425247.2012.668523.
CrossRef - Souto E. B., Nayak A. P and Murthy R. S. R. Lipid nanoemulsions for anti-cancer drug therapy. Pharmazie. 2011;66(7):473–478. http://doi.org/10.1691/ph.2011.0392.

This work is licensed under a Creative Commons Attribution 4.0 International License.





