How to Cite | Publication History | PlumX Article Matrix
Amaresh1, Guha P2, Shafat khan1 and Sumaiyah R Zari2
1Department of agriculture and food engineering Indian institute of technology Kharagpur West Bengal, 721302.
2Sher-i-Kashmir University of Agricultural Sciences and Technology Kashmir Kashmir.
Corresponding Author E-mail: amareshb142@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2458
ABSTRACT: Microwave assisted hydro-distillation (MAHD) is a new technique utilizing a modified microwave oven with Clevenger apparatus in the extraction process. MAHD was carried out to study its effect on extraction process at different power levels and leaf to water ratio. The MAHD extraction method was compared with conventional hydro-distillation (CHD) for extraction of essential oil (EO) from betel leaf (Piper betle L.) in terms of extraction yield, extraction time, energy requirement and quality of essential oil obtained. The extraction yield was generally improved by increasing microwave power level and also by increasing leaf to water ratio. The main components found by gas chromatography mass spectrometry (GC-MS) analysis were same for the oil extracted from both MAHD and CHD. There is no significant difference in the results obtained from physical properties and radical scavenging activity evaluation. MAHD was found to be more energy efficient and required less extraction time (50 minutes as compared to 210 minutes in CHD) without adversely affecting the quality of essential oil. The results obtained in this study encouraged the application of MAHD method for extraction of the essential oil. Therefore, it can be used as a good alternative method to obtain essential oils from betel leaf.
KEYWORDS: betel leaf essential oil; GC-MS; Microwave assisted hydro-distillation;
Download this article as:| Copy the following to cite this article: Amaresh A, Guha P, khan S, Zari S. R. Comparative Study of Microwave Assisted Hydro-Distillation With Conventional Hydro-Distillation for Extraction of Essential oil from Piper Betle L. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Amaresh A, Guha P, khan S, Zari S. R. Comparative Study of Microwave Assisted Hydro-Distillation With Conventional Hydro-Distillation for Extraction of Essential oil from Piper Betle L. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22837 |
Introduction
Betel leaf (Piper betle L.) is an evergreen perennial plant that belongs to family Piperaceae and is glossy in appearance, has a strong pungent aromatic flavor. There are about 100 varieties of betel leaf available in the world. About 40 varieties of betel leaf are found in India out of which, 30 are found in West Bengal (Guha, 1997; Maity, 1989; Samanta, 1994). These leaves get wasted every year due to poor post-harvest handling, packaging, and storage. Excess leaves are used as feed for cattle and sometimes buried into the ground to avoid environmental pollution and health hazards. Such wastage may be minimized by extracting the essential oil (EO) from surplus betel leaves. There are various applications of EO extracted from betel leaf in the industries as raw material for manufacturing perfumes, mouth fresheners and pharmaceuticals (Guha, 2000). It is also used as a food additive, a remedy for skin disorders and respiratory problems. Betel leaf EO has an antioxidant, antiseptic, antibacterial and antifungal properties (CSIR, 1969).
EOs are natural aromatic liquids extracted from plant material. They are immiscible with water, soluble in organic solvents (Asbahani et al, 2015). They are extracted from different plant organs such as leaves (thyme, eucalyptus), flowers (jasmine, lavender, rose), buds (clove), fruits (anise, star anise), seeds (cardamom) by several extraction methods such as hydro-distillation (HD), steam-distillation, solvent extraction (Stahl-Biskup & Sa´ez, 2002). These traditional extraction methods have low extraction efficiency and require long extraction time (Wang and Weller, 2006), lead to loss of some volatile compounds, degradation of unsaturated compounds through thermal or hydrolytic effects (Bohra et al., 1994), and there may be the presence of toxic solvent residue in the extract. These disadvantages of traditional methods led to the use of new techniques like microwave assisted extraction (MAE) (Wang et al., 2006), supercritical fluid extraction (Pourmortazavi et al., 2005), ultrasound-assisted extraction and pressurized solvent extraction (Wang & Weller, 2006; Kaufmann & Christen, 2002).
Microwaves are non-ionizing type electromagnetic waves of frequencies ranging from 300 MHz to 300 GHz. These waves can penetrate into biomaterials and generate heat by interacting with polar molecules like water (Takeuchi et al., 2009). The main benefits of microwave extractions are the reduction of extraction time and solvent used, in addition to these this process shows, even more, benefits like more efficient heating, selective heating and faster energy transfer (Chen and Spiro, 1994). Earlier published studies have successfully utilized a microwave oven for the extraction of bio-active components from plants or herbs (Ferhat et al., 2006; Lucchesi et al. 2004; Stashenko et al., 2004). Lucchesi et al. (2004) have extracted Eos from three different aromatic herbs in 30 minutes using solvent less microwave extraction system which were comparable both in qualitative as well as quantitative point of view with those obtained using CHD in 4.5 h. MAHD was developed to take advantage of microwave heating with conventional HD and used for the extraction of EOs from Lippia alba and Xylopia aromatica (Stashenko et al., 2004). Ferhat et al., (2006) has worked on MAHD extraction of EO from orange peel and found that it was better regarding extracton time (30 miutes vs 180 minutes), energy saving, product yield (0.42% vs 0.39%) and product quality.
Use of MAHD extraction of essential oil from betel leaves remained untapped in current literature. Therefore, the objectives of this work were to study the MAHD extraction of EOs from betel leaves and to compare the extraction yield/efficiency, extraction time, composition and physical properties of the EO extracted with those of HD.
Materials and Methods
Selection of Variety
A survey was conducted to study the major varieties of betel leaf available in Kharagpur region. Among two varieties (i.e., Bangla and Mitha) available, the Mitha variety was selected based on EO yield percentage as suggested in the literature (Guha, 2007). The fresh leaves of Piper betle L.var. Mitha was purchased from the market near IIT Kharagpur. Initially the leaves were rinsed with water followed by removal of petioles and were cut into pieces to increase its surface area before going to extract EO from the leaves. The initial moisture content of betel leaves was found to be 86% (w.b). The moisture contents of betel leaves were measured in duplicates according to AACC (American Association for Clinical Chemistry, 1983) method 44-19.
MAHD Apparatus and Procedure
A domestic microwave oven operated at 2.45 GHz with a maximum output power of 1000 W; variable in 100 W increments was modified for MAHD operation as shown in Fig. 2.1. The outside dimensions of the microwave oven were 311 mm × 553 mm × 482 mm. To mount the Clevenger apparatus on the flask, an opening was made on top of the oven and was fitted with a adjustable teflon socket to prevent any leakage.
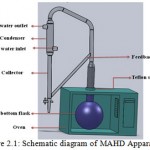 |
Figure 2.1: Schematic diagram of MAHD Apparatus |
MAHD extraction was performed at atmospheric pressure; 200g pre-treated leaves were filled in 2 liter round bottom flask with a selected amount of water (600ml, 800ml, and 1200ml). The round bottom flask was placed in the oven cavity and condenser unit was connected to the top of the round bottom flask (outside the oven) through a Teflon socket to collect extracted EO. The extraction process was started at the selected power levels of 300, 400, and 500 W (preliminary experiments showed that applying lower microwave power level takes too much time and using higher microwave power level results in degradation of EO quality and bumping effect). After extraction process was over, the apparatus was allowed to cool, and the volume of EO collected was determined. Then EO was dried with the use of anhydrous sodium sulfate and stored at 4ᵒC until analysis was done.
The yield of oil extracted is calculated by using an equation,
![]() Equation 2.1.
Equation 2.1.
Conventional Hydro Distillation (CHD)
The modified Clevenger apparatus was used for extraction of EO from betel leaves by HD method. It consists of a heating mantle fitted with a heat regulator, distillation head, 2 L round bottom flask, and condenser adapted to the coolant and clamps. A 2 L round bottom flask was filled with 200 g pre-treated betel leaves and 1000 ml of water (L/W ratio of 1:5). The cooling system was turned on to condense the evaporated liquid. The extraction process was started with slow heating and gradually increased to the maximum heating capacity of the mantle for 3.5h (i.e. until there is no further extraction). The extracted liquid was received in collector tube. After the extraction process, apparatus was allowed to cool; the volume of oil was measured.
GC-MS Analysis of Betel leaf Essential oil
A GC-MS instrument (Thermo Scientific, Trace 1300) was used to study the compositions of the EO extracted from betel leaf by both methods. A TG 5MS column of 30m length with 0.25 mm inner diameter was used. The EO samples were diluted with methanol, and a volume of 1.0 µl was injected into the GC with the injector set at 280ᵒC. He gas with a linear velocity of 1 ml min-1 was used as carrier gas. GC–MS were obtained using the following conditions: split flow 30 ml min-1; Initial oven temperature was 60 ᵒC and holding time was 1 min: then progressed from 60 to 300 ᵒC at 10 ᵒC/min; oven run time was 26 min; the ionization mode used with electronic impact at 70 eV. The compounds of the EO were identified by comparing the patterns of mass spectral fragmentation with those of similar compounds from a database library (Adams, 2007).
Physical Properties of Betel Leaf Essential oil
Specific gravity, refractive index, and appearance of the EOs extracted from betel leaves were measured according to Food Chemical Codex methods (FCC, 1996). Refractive index was measured by using Abbe refractometer at 20 ᵒC and Specific gravity was measured by using specific gravity bottle at 25 ᵒC. The EO was placed in a transparent bottle over a white background, and the color was observed with naked eye.
Radical Scavenging Method
The radical scavenging ability of betel leaf EO was measured using DPPH, a stable radical (Brand-williams, 1995). A DPPH stock solution of 0.1mM was prepared with methanol, and ascorbic acid stock solution of 100μg/ml was prepared. The ascorbic acid solution was first diluted with water and then combined with the appropriate volume of the DPPH solution to generate the standard curve. EO samples tested in duplicates. After the incubation period of 20 minutes, the solutions were read by the spectrophotometer at a wavelength of 517nm. The measured absorbance values were translated to ascorbic acid equivalents by the use of equation generated by the standard curve.
Results and Discussion
Extraction Kinetics of Mahd and CHD
Kinetics of EO extraction from betel leaf using MAHD has been compared with that of CHD in Fig. 3.1. The results obtained by MAHD at optimal conditions were compared with those of HD to evaluate the effect of microwave on the extraction of EO.
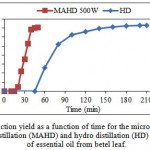 |
Figure 3.1: Extraction yield as a function of time for the microwave-assisted hydro distillation (MAHD) and hydro distillation (HD) extraction of essential oil from betel leaf. |
The time required by MAHD method to obtain first EO droplets was just 18 minutes whereas for HD it was around 45 minutes. The time taken by MAHD (50 minutes) was one-fourth of that of HD (210 minutes) for complete extraction. This is due to microwave heating involves more efficient heat flow. Unlike the conventional conductive heating methods, microwaves will heat the entire sample simultaneously at a higher rate (Kaufmann & Christen, 2002). These results are in agreement with the results obtained by Stashenko et al. (2004). They found that the extraction time required for MAHD was one-fourth of that for HD for getting almost similar extraction yield.
Extraction yield was higher for MAHD than that of HD in the early stages of extraction. The ultimate yield of EO by MAHD after 50 minutes of extraction was slightly lower than that obtained by HD after 3.5 h of extraction (1.41% vs. 1.46% w/w dry basis) but it is interesting to note that, 90% of ultimate yield (1.41% w/w dry basis) was extracted
in 50 min by MAHD whereas extraction yield was 0.24% w/w dry basis at this time for HD.
Effect of Process Parameters on Extraction Yield
Effect of Microwave Power on Extraction Yield
Effect of microwave power level (300W, 400W & 500W) on extraction yield of betel leaf EO at 0.33 L/W ratio is shown in Fig. 3.2.
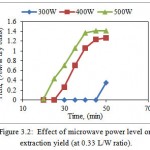 |
Figure 3.2: Effect of microwave power level on extraction yield (at 0.33 L/W ratio). |
According to Fig. 3.2, EO obtained yield after 50 minutes of extraction process for 300W, 400W, and 500W with 0.33 L/W were 0.35, 1.27 & 1.41% w/w dry basis. Extraction yield was very less when apparatus was operated at 300W. This is due to low density of microwaves at this power level. Extraction yield for 500W after 50 minutes (1.41%, w/w dry basis) of operation was about four times the extraction yield as that obtained at 300 W after 50 minutes (0.35%, w/w dry basis). The extraction yield was improved by increasing microwave power level from 300 to 500W. According to Chen and Spiro, (1994) Microwaves will be absorbed by water more intensively that eventually leads to disruption of cells which results in rapid release of vesicles containing EOs.
Effect of Leaf to Water Ratio (L/W) on Extraction Yield
The minimum solvent volume must be such that sample should immerse sufficiently. Generally, in conventional extraction methods, the performance will increase at higher solvent volume but in MAE a higher solvent volume may give lower yield (Dhobi et al., 2009). As seen in Fig. 3.3, the highest oil yield after 50 minutes was obtained by 0.33 L/W (1.41% w/w dry weight) and lowest is 0.17 L/W (0.35% w/w dry weight). It also shows that yield for 0.25 L/W at 35 minutes is equal to yield at 30 minutes for 0.33 L/W. It is clear from Fig 4.6, the yield of oil decreasing by reducing L/W ratio i.e. increasing amount of water. This was probably due to an inadequate stirring of the solvent when the microwaves are applied at larger solvent volumes. This is in agreement with Dhobi et al. (2009) where, influence of solvent to leaf ratio (30:1, 35:1, 40:1, mL/g) on the yield of silybinin was examined, and results showed that yield of silybinin decreased with the increasing solvent volume beyond 30:1.
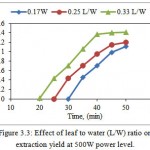 |
Figure 3.3: Effect of leaf to water (L/W) ratio on extraction yield at 500W power level. |
Characterization of Betel Leaf Essential oil by GCMS
The components of the EO were identified by GC-MS and are presented in Table 3.1 with their corresponding retention time (RT). Five major compounds were found to be estragole, chavibetol, chavicol, 4-Allyl-1,2-diacetoxybenzene and caryophyllene. Components like camphene, eucalyptol, linalool, anethole, globulol, Cubenol, τ-Muurolene, α-Cadinol were also present in trace amount. This is in agreement with Basak and Guha (2015) were, major compounds were found to be chavibetol (22.0%), estragole (15.8%), β-cubebene (13.6%), chavicol (11.8%), and caryophyllene (11.3%).
The components in betel leaf EO obtained by both HD and MAHD extraction methods were much the same, which indicate that the MAHD method did not affect its chemical composition. However, when the sample was distilled, some kinds of compounds in it may have hydrolyzed, oxidized or undergone other chemical reactions. So, there is a little difference in composition (Wang et al., 2006).
Table.3.1: Chemical compositions of betel leaf essential oil obtained by hydro distillation (HD) and microwave-assisted hydro distillation (MAHD).
| S.No | RT (min) | Compounds |
| 1 | 5.14 | Camphene |
| 2 | 6.26 | Eucolyptol |
| 3 | 7.6 | α- Linalool |
| 4 | 8.86 | Estragole |
| 5 | 9.51 | Chavicol |
| 6 | 9.54 | 2- Allylphenol |
| 7 | 10.04 | Anethole |
| 8 | 11.07 | Eugenol |
| 9 | 11.21 | Chavibetol |
| 10 | 11.89 | Caryophyllene |
| 11 | 12.66 | τ- Murelene |
| 12 | 14.00 | Globulol |
| 13 | 14.47 | Cubenol |
| 14 | 14.53 | 4-Allyl-1,2-diacetoxybenzene |
| 15 | 14.80 | α- Cadinol |
Evaluation of Physical Properties
Physical properties such as specific gravity, refractive index and appearance of betel leaf EO extracted by both HD and MAHD are shown in Table 3.2. Results obtained from this study indicated that the specific gravity and refractive indices of EO extracted by both MAHD and HD were almost similar but the color of the EO extracted by MAHD was lighter i.e. colorless to pale yellow than that obtained by HD. Mazidi et al. (2012) reported the similar results on physical properties of EO extracted from Bunium persicum Boiss.
Table 3.2: Physical properties of essential oil extracted from betel leaves.
| S.No | Physical properties | HD | MAHD |
| 1 | Specific gravity | 0.985 | 0.973 |
| 2 | Refractive index | 1.518 | 1.517 |
| 3 | Appearance | Yellow | Colorless to pale yellow |
The specific gravity of official volatile oil is approximately in between 0.842 to 1.172. The majority of the EO are lighter than water, and the results obtained in this study on specific gravity (0.985 and .973) indicates the betel leaf EO is lighter than water and its specific gravity falls within the range of specific gravity of volatile oil.
The refractive index values of volatile oil vary between 1.4600 and 1.6100 (Knevel and Digangi, 1977). Refractive index values obtained from this study are 1.518 and 1.517 (at 20 ᵒC) which are within the range of refractive indices of volatile oil.
Evaluation of Radical Scavenging Activity of Betel Leaf Essential oil
The radical scavenging activities of the betel leaf EO were evaluated using DPPH method and the results are shown in Fig. 3.4. The radical scavenging activity of EO extracted by HD was 37.82 μg/ml and those of MAHD at 300, 400, and 500 W were 36.47, 36.17, and 35.52 μg ml-1, respectively. It can be seen that EOs extracted from both methods had more or less of the same activity. There was a statistically no significant difference between the activities of both methods as determined by one-way ANOVA (F (3, 4)) = 0.64, p = 0.624).
Microwave irradiation did not cause any adverse effect on the radical scavenging activities of the extracted EOs; therefore, it can be used as a suitable alternative method to obtain EO from betel leaves.
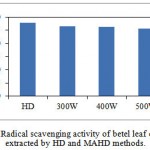 |
Figure 3.4: Radical scavenging activity of betel leaf essential oil extracted by HD and MAHD methods. |
Measurement of Energy Required for Extraction
MAHD resulted in a significant saving in the extraction time (50 min vs. 210 min) and energy required (0.4 kWh vs. 0.7 kWh) to perform the extraction. The energy needed for MAHD is about 57% that of HD. This indicates a substantial saving (43%) in the extraction cost when using MAHD instead of HD. This is in agreement with Golmakani et al., (2008) where the energy required for MAHD was 60% of that of HD.
Conclusions
High microwave power and low L/W ratio give more yield and faster extraction. This the MAHD extraction with 500W at 0.33L/W gives the highest yield in less time (1.41% w/w dry basis in 50minutes). Extraction yield was greater with MAHD than with HD during early stages of extraction. The time required by MAHD (50 minutes) was one-fourth of that of HD (210 minutes) for complete extraction.
The GC-MS analyses indicate that major components present in the EO obtained from both the methods were similar. As an excellent alternative to HD, MAHD method has no adverse effects on the composition of extracted EOs. Results obtained from physical properties evaluation indicated that the specific gravity and refractive indices of EOs obtained by both MAHD and HD were almost similar, but the color of the EOs extracted by MAHD was lighter i.e. colorless to pale yellow. Radical scavenging activity of the extracted Eos at different microwave power and HD were not significantly different (p > 0.05). So MAHD did not adversely influence the antioxidant activity.
The MAHD method offers significant advantages over HD, namely: shorter extraction times (50 min for SFME method against 3.5 h for hydro-distillation), substantial savings of energy (55%). Due to the significant reduction of time and solvent, the MAHD process is a good alternative to the extraction processes of EO from betel leaf. The results obtained in this study encourage applying the MAHD method for the extraction of the EOs of some other different herbs.
Acknowledgements
The authors acknowledge Dr. Samanta R, Assistant professor, department of chemistry IIT Kharagpur for his support. The authors also acknowledge Mr. Suradeep Basak, PhD scholar AGFE department, IIT Kharagpur for his continuous guidance during this work.
References
- A. A. C. C. Approved methods of the American Association of Cereal Chemists (8th ed.) Library of Congress, Salem, MA. 1983.
- Adams R. P. Identification of essential oil components by gas chromatography/mass spectroscopy. Journal of the American Society for Mass Spectrometry. 1997;6(8):671-672.
- El Asbahani A., Miladi K., Badri W., Sala M., Addi E. A., Casabianca H & Elaissari A. Essential oils: From extraction to encapsulation.International journal of pharmaceutics. 2015;483(1):220-243.
CrossRef - Basak S & Guha P. Modelling the effect of essential oil of betel leaf (Piper betle L.) on germination, growth, and apparent lag time of Penicillium expansum on semi-synthetic media. International journal of food microbiology. 2015;215:171-178.
CrossRef - Bohra P. M., Vaze A. S., Pangarkar V. G & Taskar A. Adsorptive recovery of water soluble essential oil components. Journal of chemical technology and biotechnology. 1994;60(1):97-102.
CrossRef - Brand-Williams W., Cuvelier M. E & Berset C. L. W. T. Use of a free radical method to evaluate antioxidant activity. LWT-Food science and Technology. 1995;28(1):25-30.
CrossRef - Chen S. S & Spiro M. Study of microwave extraction of essential oil constituents from plant materials. Journal of microwave power and electromagnetic energy. 1994;29(4):231-241.
CrossRef - CSIR (Council of Scientific and Industrial Research, New Delhi): The Wealth of India, CSIR, New Delhi. 1969;8: 84-94.
- Dhobi M., Mandal V., Hemalatha S. Optimization of microwave assisted extraction of bioactive flavonolignan. Journal of chemical metrology. 2009;34(2):13-23.
- Ferhat M. A., Meklati B. Y., Smadja J., Chemat F. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. Journal of Chromatography A. 2006;1112(1):121-126.
CrossRef - Golmakani M. T., Rezaei K. Comparison of microwave-assisted hydrodistillation withthe traditional hydrodistillation method in the extractionof essential oils from Thymus vulgaris L. Food Chemistry. 2008;109(4):925-930.
CrossRef - Guha P. Paan Theke Kutir Silpa Sambhaban (In Bengali). Exploring Betel Leaves for Cottage Industry.In: Krishi, Khadya-O- Gramin Bikash Mela –A Booklet published by the Agricultural and Food Engineering Department, IIT, Kharagpur, India. 1997;15-19.
- Guha P. Commercial exploitation of oil from betel leaves, In: Proc. Sixth Regional Workshop on Oil Seeds and Oils. IIT, Kharagpur (Ed.). Agricultural and Food Engineering Department, IIT, Kharagpur, India. 2000;56-57.
- Guha P. Betel leaf: the neglected green gold of India. J Hum Ecol. 2006;19(2):87-93.
- Kaufmann B., Christen P. Recent extraction techniques for natural products: microwave‐assisted extraction and pressurised solvent extraction. Phytochemical analysis. 2002;13(2):105-113.
CrossRef - Knevel A. L., Digangi F. F. JenkinÊs Quantitative Pharmaceutical Chem. 1977.
- Lucchesi M. E., Chemat F., Smadja J. Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydro-distillation. Journal of Chromatography A. 2004;1043(2):323-327.
CrossRef - Maity S. Extension Bulletin: The Betelvine. All India Coordinated Research Project on Betelvine, Indian Institute of Horticultural Research, Hessarghatta, Bangalore, India. 1989.
- Mazidi S., Rezaei K., Golmakani M. T., Sharifan A., Rezazadeh S. Antioxidant activity of essential oil from Black zira (Bunium persicum Boiss.) obtained by microwave-assisted hydrodistillation. Journal of Agricultural Science and Technology. 2012;14(5):1013-1022.
- Pourmortazavi S. M., Hajimirsadeghi S. S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A. 2007;2:24.
CrossRef - Samanta C. Paan chaser samasyabali-o-samadhan: Ekti samikkha (In Bengali): A Report on the Problems and Solutions of Betel Vine Cultivation. A booklet published by Mr. H. R. Adhikari, C-2/16, Karunamoyee, Salt Lake City, Kolkata-64 (WB), India. 1994.
- Sáez F., Stahl-Biskup E. (Eds.). Thyme: the genus Thymus. Taylor & Francis. 2002.
- Stashenko E. E., Jaramillo B. E & Martı́nez J. R. Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) NE Brown, grown in Colombia and evaluation of its in vitro antioxidant activity. Journal of Chromatography A. 2004;1025(1):93-103.
CrossRef - Takeuchi N., Yamanashi Y., Saito Y & Yoshikawa N. 3D simulation of superconducting microwave devices with an electromagnetic-field simulator. Physica C: Superconductivity. 2009;469(15):1662-1665.
CrossRef - Wang Z., Ding L., Li T., Zhou X., Wang L., Zhang H., Zeng H. Improved solvent-free microwave extraction of essential oil from dried Cuminum cyminum L and Zanthoxylum bungeanum Maxim. Journal of Chromatography A. 2006;1102(1):11-17.
CrossRef - Wang L., Weller C. L. Recent advances in extraction of nutraceuticals from plants. Trends in Food Science & Technology. 2006;17(6):300-312.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





