How to Cite | Publication History | PlumX Article Matrix
Swati Nayak1, 2 and Vibha Pandey1
1Department of "Plant" Pathology, Jawaharlal Nehru Krishi Vishwa Vidyalaya, Krishinagar, Adhartal, Jabalpur-482004 (M.P.), India.
2Advance Center for Plant Virology, Division of Plant Pathology, Indian Agricultural Research Institute (IARI), Pusa-110012, New Delhi, India.
Corresponding Author E-mail: swati.nayak1993@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2452
ABSTRACT: The present study was undertaken to assess the effectiveness of isolates of Trichoderma asperellum isolates against the chickpea wilt pathogen Fusarium oxysporum f. sp. ciceri. The in vitro assay revealed that there was increase in growth of test pathogen at different intervals of time but marked suppression of mycelial growth was recorded in 96 hrs under dual culture by Trichoderma asperellum isolates. There was significant growth suppression of test pathogen in culture filtrates of isolate-4 throughout the studied time compared to rest of the isolates in poison-food method. Less chlorophyll content was observed in all the culture filtrate treated plants as compared to control, in Fusarium oxysporum f. sp. ciceri (FOC) un-inoculated plants. However, after inoculation of FOC in soil, there was marked increase in chlorophyll content in culture filtrate treated plants as compared to control, with exception to isolate-8 and -10 treated plants. Minimum wilt incidence was recorded in three isolates such as isolate-1, -2 and -4 treated plants while the maximum was recorded in control. Therefore, from the present study it is concluded that the different isolates of T. asperellum showed their different abilities to check the growth of pathogenic fungi under in vitro and in vivo conditions.
KEYWORDS: Chickpea; Chlorophyll content Disease incidence;FOC; Trichoderma asperellum;
Download this article as:| Copy the following to cite this article: Nayak S, Pandey V. Evaluation of Rhizosphere Isolates for Management of Fusarium oxysporium f. sp. ciceri of Chickpea (Cicer arietinum L.). Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Nayak S, Pandey V. Evaluation of Rhizosphere Isolates for Management of Fusarium oxysporium f. sp. ciceri of Chickpea (Cicer arietinum L.). Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=21730 |
Introduction
Fusarium wilt is one of the major diseases of chickpea and at national level, the yield losses encountered was reported to the tune of 60 per cent.1 The excessive misuse of a wide range of chemical fungicides is being used to suppress the disease but these chemicals have a negative impact on human health and are hazardous to the environment.2 The better alternatives to chemicals are the microbes such as Trichoderma spp. residing in the rhizosphere of crop plants that have the ability to suppress the pathogens growth.3 Utilization of resident mycoflora of any crop will be helpful in plant health management as these mycoflora produces several secondary metabolites that act against pathogenic microbes and produces other plant growth promoting substances for growth of crop. There were many reports on bio-control agents for control of Fusarium wilt pathogen; some bioactive compounds that were extracted from antagonistic fungi have been found to inhibit Fusarium wilt of tomato and brinjal.4 The application of Trichoderma species can control a large number of soil-borne fungi i.e. Fusarium spp., Rhizoctonia solani, Pythium spp., Sclerotium rolfsii in vegetables, fruit and industrial crops.5 Trichoderma is directed to achieve effective mycoparasitic strains as biocontrol agents against plant fungal pathogens under a wide range of adverse environmental conditions.6 Therefore, the present study was undertaken with an aim (i) to evaluate the inhibition potential of different isolates of bioagents under in vitro conditions, and (ii) to establish their effect on management of Fusarium wilt apart physiological properties of crop under field conditions.
Material and Methods
Collection of diseased specimens and purification of the pathogens
Diseased chickpea plants exhibiting typical symptoms of wilt incidence levels were collected from the experimental field of AICRP on chickpea, Jawaharlal Nehru Krishi Vishwa Vidyalaya (22°49’- 22° 80’N; 78°21’- 80°58’E), Jabalpur in the Central India during 2015-16. The pathogen was isolated and further purified through hyphal tip method and sub-cultured on Potato Dextrose Agar (PDA) containing plates at 4°C for further use. Dilution plate method was used to isolate the Trichoderma asperellum isolates from soil samples of chickpea plant showing different level of wilt symptoms on Rose Bengal Agar (RBA) medium. Plates with RBA medium was added with 0.1 mL (=10-4) of suspension and incubated at 22 ± 2°C for 15 days. The colonies were transferred to test tubes containing PDA medium. The Trichoderma asperellum isolates were designated as TS1, TS2, TS3, TS4, TS5, TS6, TS7, TS8, TS9 and TS10 throughout the study.
Evaluation of antagonistic potential of beneficial fungi in vitro
The antagonistic potentials of Trichoderma asperellum isolates were evaluated against the F. oxysporum through dual culture technique.7 A five mm disc of different fungal isolates was cut out from the seven days old culture and placed close to one end of the Petri-plate containing 20 mL solidified PDA medium. At the opposite end, a similar disc from the culture of the pathogen F. oxysporum was placed simultaneously. The Petri-plates were incubated at 25±2°C in a BOD incubator (HAMCO-42, Hindustan Apparatus Mfg. Company, Mumbai) and inhibition of the growth of the pathogen by the antagonistic fungi was measured after 48, 72, and 96 hrs of incubation till both occupies the entire space of Petri-plate.
Culture filtrate of Trichoderma asperellum isolates were grown in PDA broth for 10 days were collected after passing it twice through Whatman filter paper No. 1 (90 mm Ø). These filtrates were used to amend Petri-plates containing PDA at 5 per cent concentration and incubated at 25±2°C and observations were recorded after 48, 72, 96, 120, 144 and 168 hrs, respectively; an un-amended Petri-plate served as check (control). Each treatment was replicated thrice and the experiment was conducted twice with same experimental set-up. The antagonism was measured on the basis of per cent inhibition of the pathogen by the bioagent using following formula:
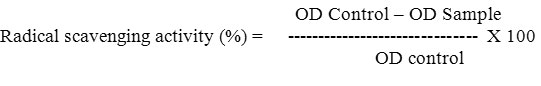
Assessment of Antagonistic Potential of T. Asperellum Isolates under in vivo Conditions
The inoculum of FOC-fungus was produced on sand + wheat flour mix (9:1), moistened with water and autoclave twice for 90 min on two consecutive days. Thirty days after sowing of the seeds, the culture filtrate of individual beneficial fungi were added into the pots that were already containing the FOC inoculum spread on sand + wheat flour mix (@ 5 gm/ kg of potting mix. Two sets of experiments with three replications for each treatment were maintained. Ten-chickpea seeds were sown in each clean pot at a depth of 2-3 cm in six pots for each strain of T. asperellum along with un-inoculated control.
Measurement of relative water content (RWC)
Measurements of RWC were performed on leaves collected from chickpea plants according to Barrs and Weatherly.8 Initially, the individual leaves were removed from the stem with tweezers and weighed immediately (fresh mass; FM) to obtain a minimum 0.5 gm from each treatment sample. In order to obtain the turgid mass (TM), leaves were floated in distilled water inside a closed Petri-dish. At the end of the imbibition period, leaf samples were placed in a pre-heated Hot air oven (SGS, Huanghua Guangming Instrument Co., Ltd., Huanghua, China) at 80°C for 48 hrs to obtain the dry mass (DM). The values of FM, TM, and DM were used to calculate RWC using the following equation:
RWC (%) = [(FM – DM)/ (TM – DM)] × 100
Chlorophyll content index (CCI)
Chlorophyll content index was estimated through the portable chlorophyll meter.9 Fully expanded leaf sample from three places of each plant of different treatments were selected for estimation of chlorophyll content index. Triplicate readings of chlorophyll content index of individual plant leaves were taken before and after inoculation (BI and AI, respectively) of FOC in pots those were already inoculated with isolates of Trichoderma asperellum. The mean of triplicate readings using SPAD-502 (SPAD-502; Minolta, Japan) around the midpoint near the midrib of each sample were recorded for different treatments of chickpea leaf and average was calculated. The values obtained for AI and BI were used to calculate chlorophyll loss per cent using the following equation:
CCI (%) = [(BI – AI)/ (BI)] × 100
Disease incidence
The per cent wilt disease incidence of each treatment was calculated by using following formula:

Results
Evaluation of efficacy of T. asperellum isolates against Fusarium oxysporum f. sp. ciceri under in vitro and in vivo conditions
Diseased chickpea roots (Fig. 1A) exhibiting symptoms of wilt incidence levels were collected, futher pathogen was isolated by hyphal tip method. B) Isolated Fusarium oxysporum f. sp. ciceri was inoculated on PDA containing media plates and then plated and sub-cultured on Potato Dextrose Agar (PDA) containing plates and the pathogen Fusarium oxysporum f. sp. ciceri was isolated and sub-cultured on Potato Dextrose Agar (PDA) containing plates (Fig. 1B). The dominant rhizosphere mycoflora of Trichoderma asperellum was obtained on Rose Bengal Agar (RBA) medium (Fig. 1C). The effect of ten isolates of T. asperellum were tested and found highly suppressive towards the test pathogen (Table 1). The suppression of mycelial growth of FOC by different isolates of T. asperellum varied between 17.90 mm to 24.78 mm. The highest (17.90 mm) inhibition was recorded with isolate-10 while least (24.78 mm) with isolate-9. The isolate-2 (21.10 mm), -3 (21.07 mm), -5 (21.07 mm), -6 (21.88 mm) and -8 (21.05 mm) were equally suppressive towards the test pathogen (Fig. 2) and were next best to isolate-4 (20.30 mm) and -7 (20.91 mm). Although, there was increase in growth of pathogen at each time interval but marked suppression of mycelial growth was recorded in 96 hrs. Isolate-4 (except in 48 hrs) and -1 was highly suppressive throughout the studied time.
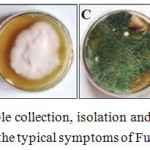 |
Figure 1: Fusarium pathogen sample collection, isolation and sub-culturing on media plates. A) diseased chickpea plants showing the typical symptoms of Fusarium wilt disease were collected. |
The pathogen was isolated by hyphal tip method for the test pathogen and dilution plate method for Trichoderma isolates. B) The isolated Fusarium oxysporum f. sp. ciceri was inoculated on PDA containing media plates and cultured at 27-28°C for 72hrs. C) The isolated root pathogen sample was serially diluted and cultured on RBA medium at 27-28°C for 48hrs. The growth of different rhizosphere mycoflora observed in the plates along with Trichoderma asperellum (green colour) as a dominant.
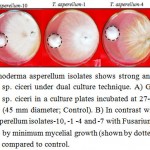 |
Figure 2. Different Trichoderma asperellum isolates shows strong antagonistic effect against Fusarium oxysporum f. sp. ciceri under dual culture technique. A) Growth of test pathogen-Fusarium oxysporum f. sp. ciceri in a culture plates incubated at 27-28ºC for 96 hrs without Trichoderma asperellum (45 mm diameter; Control). B) In contrast with this, the dual culture assay of Trichoderma asperellum isolates-10, -1 -4 and -7 with Fusarium oxysporum f. sp. ciceri shows antagonistic effect by minimum mycelial growth (shown by dotted circle) after incubation at 27-28°C for 96 hrs as compared to control. |
Table 1: Screening of Trichoderma asperellum isolates against FOC through dual culture method
| Trichoderma asperellum (growth in mm) | |||||
| 48 hrs | 72 hrs | 96 hrs | Mean | ||
| TA1 | 22.51(14.67) | 19.33(11.00) | 15.26(7.00) | 19.03 | |
| TA2 | 27.34(21.17) | 20.68(12.50) | 15.28(7.17) | 21.1 | |
| TA3 | 25.46(18.50) | 22.65(14.83) | 14.92(6.67) | 21.01 | |
| TA4 | 26.36(19.83) | 20.23(12.00) | 14.30(6.17) | 20.3 | |
| TA5 | 23.18(15.50) | 20.54(12.33) | 19.5(11.17) | 21.07 | |
| TA6 | 28.30(22.50) | 20.82(12.67) | 16.52(8.17) | 21.88 | |
| TA7 | 26.67(20.17) | 21.97(14.00) | 14.09(6.00) | 20.91 | |
| TA8 | 26.18(19.50) | 21.37(13.33) | 15.61(7.67) | 21.05 | |
| TA9 | 32.04(25.50) | 24.84(17.67) | 17.45(9.00) | 24.78 | |
| TA10 | 22.70(14.93) | 17.45(9.00) | 13.56(5.50) | 17.9 | |
| Control | 22.81(15.00) | 33.20(30.00) | 42.13(45.00) | 32.71 | |
| Mean | 25.78 | 22.1 | 18.06 | ||
| CV | 7.7 | ||||
| hrs CD (P≤ 0.05) | 0.83 | ||||
| Fungus CD (P≤ 0.05) | 1.59 | ||||
| Fungus × hrs | 2.76 | ||||
*The values in the parenthesis are original value.
In poison-food method, highest (35.54 mm) inhibition was recorded in culture filtrate of isolate-4 of T. asperellum followed by isolate-5 (39.22 mm) and isolate-9 (39.70 mm) (Table 2). Although the growth of pathogen had increased with increase in time but increase was comparatively slower after 120 to 144 hrs. The marked growth suppression of test pathogen in culture filtrates of isolate-4 was recorded throughout the studied time as compared to rest of the isolates.
Table 2: Evaluation of efficacy of different isolates of Trichoderma asperellum against Fusarium oxysporium f. sp. ciceri under in vitro conditions
| Isolates | Trichoderma asperellum (growth in mm) | ||||||||||
| 48 hrs | 72 hrs | 96 hrs | 120 hrs | 144 hrs | 168 hrs | Mean | |||||
| TA1 | 30.4 | 34.1 | 41.00(43.00) | 50.80(60.00) | 51.9(62.00) | 64.7(81.60) | 45.48 | ||||
| TA2 | 30.4 | 34.5 | 38.80(39.30) | 49.50(57.80) | 55.3(67.60) | 65.7(83.00) | 45.71 | ||||
| TA3 | 27.5 | 31.7 | 38.10(38.00) | 45.90(51.50) | 51.7(61.50) | 55.1(67.30) | 41.65 | ||||
| TA4 | 26 | 29.1 | 34.20(31.60) | 38.80(39.30) | 40.1(41.50) | 45.0(50.00) | 35.54 | ||||
| TA5 | 26.6 | 31.3 | 35.70(34.00) | 39.60(40.60) | 45.4(50.60) | 56.8(70.00) | 39.22 | ||||
| TA6 | 25.3 | 30.2 | 37.30(36.60) | 50.40(59.30) | 51.6(61.30) | 55.6(68.00) | 41.71 | ||||
| TA7 | 29.1 | 31.7 | 41.70(44.30) | 56.40(69.30) | 63.0(79.30) | 64.4(81.30) | 47.72 | ||||
| TA8 | 28.3 | 31.30(27.00) | 42.10(45.00) | 46.20(52.10) | 56.8(70.00) | 67.2(85.00) | 45.33 | ||||
| TA9 | 26.2 | 31.10(26.00) | 36.10(34.60) | 41.90(44.60) | 46.1(52.00) | 56.8(70.00) | 39.7 | ||||
| TA10 | 23.8 | 30.90(26.30) | 42.50(45.60) | 59.50(74.10) | 62.0(78.00) | 64.0(80.80) | 47.11 | ||||
| Control | 22.81 | 33.20(30.00) | 42.13(45.00) | 49.60(58.00) | 51.94(62.00) | 53.71(64.96) | 42.23 | ||||
| Mean | 26.94 | 31.74 | 39 | 48.05 | 52.35 | 59 | |||||
| C V | 2.15 | ||||||||||
| Fungus CD (P≤ 0.05) | 0.6 | ||||||||||
| Hours CD (P≤ 0.05) | 0.44 | ||||||||||
| Fungus × hrs | 1.47 | ||||||||||
*The numbers in the parenthesis are original values.
Effect of T. asperellum isolates on the physiological parameters and disease incidence of crop
The effect of culture filtrate (Fig. 3) treatment on relative water content and disease incidence was studied under poly-house condition (Table 3). There was significant increase in relative water content of chickpea leaves, inoculated with culture filtrate of different isolates of T. asperellum over control with exception to isolate-3. The range varied between 42.99 to 63.43 per cent. The highest RWC was recorded in isolate-2 followed by isolate-1 and isolate-4. The RWC of isolate-5, -7 and -10 were statistically at par with each other. Similarly, there was negligible difference in RWC of plants treated with isolate-6, -8 and -9. Chlorophyll content index of chickpea leaves ranged 44.10 to 36.65 per cent in un-inoculated FOC while 30.21 to 40.0 percent in FOC inoculated (treated with bioagent) plants. The highest (44.10%) chlorophyll content was recorded in control while least (30.21%) was recorded in after FOC inoculation. The Chlorophyll loss percent was maximum (31.50%) in control, while minimum (1.25%) in T. asperellum isolate-9 treated plants. The minimum wilt incidence was recorded in isolate-1(19.35%), isolate-2 (19.41%) and isolate-4 (19.46%), while the maximum (57.67%) was recorded in the control. The effect of treatments on wilt incidence of isolates-7, -5 and -10 (26.54%, 29.99% and 29.99%, respectively) were equally suppressive against FOC.
 |
Figure 3: Variations in the culture filtrates from the Trichoderma asperellum isolates. Ten Trichoderma asperellum isolates were grown in Potato Dextrose broth for 10 hrs. The culture filtrate from each isolate (1-10) has difference in colour, this property of culture filtrate might be attributed to the diversity in metabolic content among the isolates.
|
Table 3: Effect of different Trichoderma asperellum isolates on physiological parameters and disease incidence of crop
| T. asperellum | RWC
(%) |
CCI (SPAD 502) | Chlorophyll
loss (%) |
Wilt incidence (%) |
||||
| BI AI | ||||||||
| TS1 | 62.04 | 41.93 | 41.16 | 1.84 | 19.35 | |||
| TS2 | 63.43 | 37.13 | 35.86 | 3.42 | 19.41 | |||
| TS3 | 38.05 | 42.59 | 40.20 | 5.61 | 35.24 | |||
| TS4 | 60.90 | 41.35 | 40.38 | 2.35 | 19.46 | |||
| TS5 | 58.27 | 42.20 | 37.73 | 10.59 | 29.99 | |||
| TS6 | 51.94 | 40.21 | 33.62 | 16.39 | 45.00 | |||
| TS7 | 59.35 | 38.89 | 37.39 | 3.86 | 26.54 | |||
| TS8 | 54.34 | 41.03 | 27.82 | 32.20 | 39.22 | |||
| TS9 | 51.94 | 38.36 | 37.88 | 1.25 | 45.00 | |||
| TS10 | 58.06 | 35.80 | 30.62 | 14.47 | 29.99 | |||
| Control | 42.99 | 44.10 | 30.21 | 31.50 | 57.67 | |||
| CV | 2.20 | 3.33 | 4.27 | 2.66 | ||||
| CD (P≤0.05) | 2.05 | 2.29 | 2.59 | 1.51 | ||||
*BI- Before Inoculation of FOC and AI – After Inoculation of FOC.
Discussion
All the ten isolates of T. asperellum had shown strong antagonistic effect against FOC under dual culture. The higher antagonistic activity of all the isolates of T. asperellum against the test fungi could be due to their fast mycelia growth and competition for nutrients in growing medium. Member of Trichoderma species are known for their active hyperparasites of several soil fungi and hence they are used as a biocontrol agents.10 Akrami and co-workers11 found that three isolates (T. harzianum, T. asperellum, T. virens) were effective against Fusarium rot of lentil. Whereas, Prasad et al.12 observed two antagonistic fungi viz., T. harzianum (PDBCTH-10) and T. viride (PDBCTV) against Fusarium oxysporium f. sp. ciceri. In poison food technique, the highest inhibition (35.54 mm) was recorded in isolate-4 that slower down the growth of test pathogen throughout the studied time. The second and equally suppressive isolate-5 (39.22 mm) and -9 (39.70 mm) while the rest of the isolate promoted the growth of FOC. It might be due to competition for food and space. Competition for carbon, nitrogen and iron mechanism associated with biocontrol has been proposed by Couteadier.13 Metabolite of T. harzianum, T. viride and T. virens have been found to inhibit the mycelial growth of Fusarium oxysporum f. sp. ciceri causing wilt disease in chickpea.14 Trichoderma strains inhibit the infections caused by plant pathogens using different biocontrol mechanisms like competition, antibiosis, mycoparasitism, hyphal interactions, and enzyme secretion.15,16 The minimum chlorophyll content index and relative water content was recorded in pot treated with FOC in control while higher in others, treated with culture filtrate of T. asperellum isolates. The range of RWC varied between 42.99 to 63.43 per cent. The highest RWC was recorded in isolate-2 followed by isolate 1and isolate-4. The RWC of isolate-5, -7 and -10 were statistically at par with each other. Chlorophyll content of chickpea leaves ranged between 44.10 to 37.13 in before FOC treated pots. Less chlorophyll content was observed in all the culture filtrate treated plants compared to control in non FOC inoculated plants. However, after inoculation of FOC in soil there was marked increase in chlorophyll content in treated plants with culture filtrate compared to control, with exception in isolate-8 and-10. The highest chlorophyll content was recorded in isolate-1 followed by isolate-4, isolate-3 that were equally effective on plant.
The minimum wilt incidence was recorded in isolate-1, isolate-2 and isolate-4, while the maximum was recorded in control. This result might be attributed to the potential of beneficial mycoflora to overcome the biotic stresses by preventing the pathogenic fungi to colonies the root system and further clogging of xylem vessel to create water stress. The water stress resulted in significant a decrease (55%) in chlorophyll content and the leaf relative water content was recorded by Kirnak and others.17 Although the minimum wilt incidence was recorded in Aspegillus niger isolate-3 (18.04%) but isolate-1, -2 and -8 equally (29.99%) suppressed the disease and were next best to the isolate-3 against the FOC. Plants pre-treated with FOC followed by beneficial fungus appeared healthy with no wilting or root rot symptoms for more than 10 days. Wilting symptoms can be observed within 25 days of sowing into infected soil.18 According to Heydari and Pessarakli19 different mode of action of bio control active micro-organism in controlling fungal plant disease include hyper-parasitism, predation, antibiosis, cross protection, competition for site and nutrient and induced resistance.
Conclusion
Based on our results, chickpea root system contains biological diversity even under stress conditions to counter the effect of more vulnerable plant disease, such as Fusarium wilt. Immediate actions through metabolic active bioagents are necessary to restore the balance of the soil ecosystem and plant health.
References
- Singh G., Chen W., Rubiales D., Moore K., Sharma Y. R., Gan Y. Disease and their management. In:Chickpea Breeding and Management (Yadav R., Chen W., Sharma Y. R., ed). CABI publishers. 2007;497.
CrossRef - Ozgonen H., Bicici M., Erkilic A. The effect of salicyclic acid and endomycorrhizal fungus Glomus etunicatum on plant development of tomatoes and Fusarium wilt caused by Fusarium oxysporum sp. lycopersici. Turk. J. Agric. For. 2001;25:25-9.
- Fravel D., Olivian C., Alabouvette C. Fusarium oxysporum and its biocontrol. New Phytopathol. 2003;157:284-7.
CrossRef - Khan Z., Kim Z., Kim Y. H., Kim S. G., Kim H. W. Observation of the suppression of root-knot nematode (Meloigogyne arenaria) on tomato by incorporation of cyanobacteria power (Oscillatoria chlorine) into potting filed soil. Bioresource Tech. 2007;98:69-73.
CrossRef - Ngo B. H., Vu D. N., Tran D. Q. Analyze antagonist effects of Trichoderma for controlling southern stem rot caused by Sclerotium rolfsii on peanut. Pl. Prot. 2006;1:12-4.
- Manczinger L., Antal Z., Kredics L. Ecophysiology and breeding of mycoparasitic Trichoderma strains (a review). Acta Microbiol Immunol Hung. 2002;49:1-14.
CrossRef - Dennis C., Webster J. Antagonistic properties of species groups of Trichoderma Production of non-volatile antibiotics. Trans. Br. Mycol. Soc. 1971;57:41-8.
CrossRef - Barrs H. D., Weatherly P. E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. J. Biol. Sci. 1962;15:413-28.
CrossRef - Peng S., Garcia F., Laza R., Cassman K. G. Leaf thickness affects the estimation of leaf nursing a chlorophyll meter. Rice Res. Newsletter. 1992;17(16):19-20.
- Ekefan E. J., Jama A., Gowen S. R. Potential of Trichoderma harzianium isolates in biocontrol of Colletotrichum capsici causing anthracnose of pepper in Nigeria. Appl. Bioscience. 2009;20:1138-45.
- Akrami M., Golzary H., Ahmadzadeh M. Evaluation of different combinations of Trichoderma species for controlling Fusarium rot of lentil. J. Biotechnol. 2011;10:2653-8.
- Prasad R. D., Rangeshwaran R., Anuroop C. P., Rashni H. J. Biological control of wilt and root rot of chickpea under field conditions. Plant. Protect. Sci. 2002;1:72-5.
- Couteaudie Y. Competition for carbon in soil and rhizosphere a mechanism involved in biological control ofFusarium In: Biological Control of Plant Diseases (Tjamos ES. et al.). New York Plenum Press. 1992;99-104.
CrossRef - Dubey S. C., Suresh M., Singh B. Evaluation of Trichoderma species against Fusarium oxysporum sp. ciceris for integrated management of chickpea wilt. Biol. Control. 2007;40:118-27.
CrossRef - Hajieghrari B., Torabi-Giglou M., Mohammadi M. R., Davari M. Biological potential of some Iranian Trichoderma isolates in the control of soil borne plant pathogenic fungi. J. Biotechnol. 2008;7(8):967-72.
- Poovendran P., Kalaigandhi V., Parivuguna V. In vitro study of antagonistic effect of Trichoderma, on tea plant pathogen, Phomopsis theae. Arch. Appl. Sci. Res. 2011;3(4):352–358.
- Kirnak H., Kaya C., Tas I., Higgs D. The influence of water deficit on vegetative growth, physiology, fruit yield and quality in eggplants. Bulgarian J. Plant Physiol. 2001;27(3-4):34-46.
- Nene Y. L., Haware M. P., Reddy M. V. Diagnosis of some wilt-like disorders of chickpea (Cicer arientium) ICRISAT Information bulletin. 1978;3:44.
- Heydari A., Pessarakii M. A. R. Review on biological control of fungal plant pathogens using microbial antagonists. Biol. Sc. 2010;10(4):273-290.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





