How to Cite | Publication History | PlumX Article Matrix
Evidence Based Comparative Studies on free and Immobilized Therapeutic Protein
Rahamat Unissa, Ashwini Kumari Chauhan , Asmathpet Malleshwari, Narsareddy Vinitha, Karra Haarika, Chundru Sri Aneesha, Datti Navya Teja Sree, Mounika Sangishetty and Ishrath Begum
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Malla Reddy College of Pharmacy, Maisammaguda, Dhulapally, Secunderabad, Osmania University, Telangana, India.
Corresponding Author E-mail: srunissa@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2451
ABSTRACT: The aims of the present work were to carry out comparative studies on free and immobilized l-arginine deiminase obtained from marine Vibrio alginolyticus 1374. Therapeutic proteins of microbial origin though possess excellent pharmacological activities gets quickly broken down into amino acids by the action of proteases in the body. Hence to protect its activity and integrity the enzyme was attached with a compatible polymer such as PEG and was further evaluated for the presence of the stability and activity. Enzyme production was carried out by culturing Vibrio sp. under optimal conditions and was purified by standard chromatographical techniques. The purified enzyme thus obtained was immobilized using different concentrations of PEG20k and purified. The Pegylated enzyme was kinetically characterized and evaluated for anti-proliferating activity against four cancer cell lines. mPEG-Succinimidyl Succinate, MW 20,000 coupled with ADI with very high affinity under mild conditions. The pegylated ADI had 3.1 PEG chains of 20k length which not only protected the enzyme from degradation but also increased plasma t ½ and prevented immunogenicity. It showed approximate molecular weight of about 112 k Da. The Km and Vmax values of PEGylated ADI were found to be 2.94 ± 0.13 mM and 129.87 ± 1.24 U/ml/min respectively. Though there was a slight change in Km and Vmax values, it still retained its activity against four cancer cell lines with slight decrease in the activity compared to free enzyme. L-Arginine deiminase of Vibrio sp. remained effective even after pegylation. Hence it can be a promising candidate in treatment of l-arginine auxotrophic cancers. In vivo studies must be carried out to develop the enzyme in the form of chemotherapeutic drug.
KEYWORDS: A375-C6; argininosuccinate synthetase; clone E6-1cell lines HCT-113 and jurkat;MCF-7; ornithine carbamoyl transferase; Vibrio alginolyticus 1374;
Download this article as:| Copy the following to cite this article: Unissa R, Chauhan A. K, Malleshwari A, Vinitha N, Haarika K, Aneesha C. S, Sree D. N. T, Monika S, Begum I. Evidence Based Comparative Studies on free and Immobilized Therapeutic Protein. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Unissa R, Chauhan A. K, Malleshwari A, Vinitha N, Haarika K, Aneesha C. S, Sree D. N. T, Monika S, Begum I. Evidence Based Comparative Studies on free and Immobilized Therapeutic Protein. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22754 |
Introduction
Cancer is a disease characterized by abnormal proliferation of cells. It is one of the leading causes of death in the world, and it is of serious problem to people living in both the developing as well as developed nations.1 Cancer can be cured without much problem if it is diagnosed at the earlier stages. Nutritional starvation of cancer cells have been tried by many researchers and have been found to be successful in a many cases. Excellent example of which is l-asparginase presently used in the treatment of leukemia. It is available in the market as oncaspar.2
Another nonessential amino acid in humans is l-arginine, which is synthesised from citrulline using two enzymes, argininosuccinate synthetase (ASS) and argininosuccinate lyase (ASL). ASS converts citrulline to argininosuccinate, which (in turn) is converted to arginine by ASL. Cells that do not express ASS can be sensitive to arginine-depleting enzymes such as arginine deiminase (ADI) and l-arginase. Several cancer cells have been found to lack ASS expression and hence are auxotrophic for arginine. Hence arginine depletion can be effective in treating these tumours and possibly other tumours.3
Similar to L-asparaginase, ADI is also derived from microorganisms. It is not produced by mammals such as humans. Consequently, the enzyme has a very short circulating half-life and is immunogenic. Due to the fact that these enzymes are foreign proteins, multiple injections are needed to produce the desired effect of arginine deprivation. A method of increasing a drug’s circulating half-life and decreasing its immunogenicity is to link the drug with PEG. Moreover pegylation also protects the drug against degradation by proteolytic enzymes. A number of other drugs have been formulated with PEG. These include (for example) PEG-interferon and asparaginase.4,2 The latter drug is also derived from microbes and is an enzyme that has been used successfully in leukaemia.
Even though pegylation is considered as a noval approach for delivering wide range of therapeutic proteins, it was seen that most of the proteins loose their activity upon pegylation.
In our preliminary studies we have isolated a potential enzyme producer from marine sources and used the same for the optimal production of the enzyme.5,6 In our another article we have explained purification process and kinetic characterization of the enzyme. The purified enzyme showed a good activity against various cancer cell lines.7 Here in this present study we have formulated the enzyme with PEG20K and further evaluated its characteristics.
Materials and Methods
Chemicals and Reagents
The reagents employed in the current investigation were of high grade and quality. Media components used for the preparation of bacteriological medium were of Hi- Media Labs. Remaining chemicals belongs to Sigma Aldrich.
Preparation of ADI
Production of ADI was carried out using marine Vibrio alginolyticus 1374 under optimal conditions and was purified by standard purification methods.8,7
Enzyme Assay
Citrulline concentration was used to measure enzyme activity colorimetrically using a modified version of the method described by Oginsky9,7
Total Protein
The concentration of the protein present in the sample was determined by Lowry’s method by taking absorbance values at 660 nm and the values were expressed in mg
Modification of Enzyme
Pegylation of ADI and its Characterization
An ADI solution of 0.5 mg/mL was prepared in 50 mM PBS buffer, pH 7.4. PEG reagents were added into the ADI solution to a molar ratio of PEG to ADI of 100:1, 50:1 or 20:1. The PEGylation reaction was carried out for 3 h or 24 h at room temperature, with magnetic stirring. The reaction mixture was washed 3 times repeatedly with PBS (20 mM, pH 7.4) through a 50 k Da Amicon Ultra-50 to remove free PEGs. When non-PEGylated ADI was detected on SDS-PAGE, the concentrated PEG-ADI was further purified by anion-exchange chromatography.
Molecular Weight Analysis of ADI and ADI-PEG
The products of pegylation were analyzed by automated gel electrophoresis.
Effect of p H on the free and Pegylated ADI
The stability of free and pegylated ADI were tested at different p H values by maintaining them in the following buffer solutions for one hour : for p H 6-7 phosphate buffer ,for p H 8-9 borate buffer, for p H 10 Tris HCl buffer. The enzymatic activities of the free and the pegylated ADI were tested after one hour of incubation .The p H at which the free enzyme showed maximum activity was considered to possess 100% residual activity and the activities of the other p H values were evaluated with respect to the highest value of the residual activity.
Thermal Stability of Purified free and Pegylated Enzyme
To check the thermal stability, purified enzyme was incubated at various temperatures (25, 30, 35, 37, 40, 50, 60 and 700C) for 1 hour and then enzyme activity was observed.
Determination of Half-life of free and Immobilized ADI
The half-life of free and immobilized ADI was determined in vitro using PBS (p H 7.4) buffer. Solutions of the immobilized and free enzyme were slowly homogenized and incubated at 370C to measure the half-life of both. At the intervals of 1, 3, 6 and 24h, a sampling was done and the enzyme activity was determined.
In Vitro Cytotoxicity Studies
Cell Line and Culture
Cancer cell lines were obtained from Tata Memorial institute of cancer research, Mumbai, India. The cells were maintained in RPMI 1640 medium at 37°C.
In Vitro Assay for Cytotoxicity Activity (MTT Assay)
The effect of samples on cell lines were tested by MTT assay.10 Cancer cells were cultured in 96 welled plate containing 5ml of the medium and incubated for 2 days to attain optimal growth. Then the drug was added into the wells to reach a concentration of 0, 0.001, 0.01, 0.1, 0.5, 1, 10 and 100 U/ml respectively and incubated at 37°C.The medium was vacuumed off and the cells remained were washed with PBS solution. To that 1ml of MTT reagent was added and incubated for 3-4hrs.Formazan crystals thus formed were dissolved in isopropyl alcohol and absorbance was determined at 570nm.Enzyme activity was expressed in terms of IC50.
% Cell viability = A570 of treated cells / A570 of control cells × 100 %.
Results and Discussion
Pegylation of ADI and its Characterization
Moelcular weight of free ADI was found to be 48 k Da in our previous studies. N-hydroxylsuccinimide (NHS) active esters, such as mPEG-SS are the generally used derivatives for lysine attachments. Therefore, the mPEG-SS was used for the PEGylation of ADI in the present work. Reaction between the Ɛ-amino group of lysine(s) or the N-terminal amine and the NHS ester generates a physiological strong amide linkage. Generally, increasing pH increases the speed of reaction and lowering pH reduces rate of reaction. In this case, PEGlytion reactions were performed at physiological pH (7.4) and room temperature. To optimize the reaction time and the quantity of mPEG-SS required for complete PEGylation of ADI, the purified ADI was incubated with molar ratio of 1: 20, 1: 50, and 1: 100 mPEG-SS concentrations with two different incubation times (3 h and 24 h). The products of PEGylation of ADI were resolved by SDS-PAGE, which showed that the MW of ADI after PEGylation was increased compared to non-PEGylated ADI. After PEGylation, a mixture of PEG-ADI with varied molecular weights was obtained (smear on the SDS PAGE) due to the size distribution of PEG molecules. Less non-PEGylated ADI was observed from the molar ratio of 1: 100 and 1: 50 (ADI: mPEG-SS) than 1: 20 (Figure 2). Furthermore, different reaction times of 3 h and 20 h did not influence the yield of PEGylation (Figure 2, lane 1 compares to lane 2, lane 5 compares to lane 6). From these results, the optimum ADI : PEG ratio (molar) and reaction period were found to be 1: 50 and 3 h, respectively.
Non-PEGylated ADI was still detectable on SDS-PAGE. Therefore, the solution of ADI after PEGylation was concentrated (through a 50 k Da Amicon Ultra-50) and further purified by anion-exchange chromatography. PEG-ADI did not bind to the column due to the covered charges by PEG molecules; thereby it was obtained from the flow through (Figure 1). The PEG-ADI and ADI were tested by gel electrophoresis system. PEGylated ADI was well separated from the mixture by anion-exchange chromatography, because there was no non-PEGylated ADI noticed (see Figure 2, lane 1) from the sample of purified PEGylated ADI.
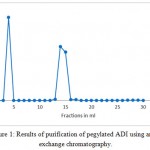 |
Figure 1: Results of purification of pegylated ADI using anion exchange chromatography.
|
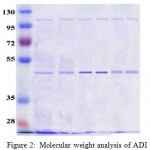 |
Figure 2: Molecular weight analysis of ADI and ADI-PEG
|
The approximate molecular weight of PEGylated ADI was determined by automated gel electrophoresis system. Predicted number of PEG molecule linked to Lys was determined by this equation: Number = [MW of ADI-mPEG-SS) – MW of (ADI, 48 KDa)] / MW of (mPEG-SS, 20 K Da). The mean molecular weight of ADI-PEG-20 was found to be 112 k Da. The predicted number of PEG molecules bound to lysine was found to be 3.1.
Kinetic Analysis of PEGylated ADI
The kinetic data of Km and Vmax values of PEGylated ADIs were found to be 2.94 ± 0.13 mM and 129.87 ± 1.24 U/ml/min respectively. The Km value of the ADI was found to be slightly increased upon pegylation, which suggested that the attached PEG molecules on the surface of the enzyme did not significantly reduce the affinity for the substrate. Free ADI was found to have high substrate affinity when compared to immobilized ADI. The results obtained in our study coincides with that of Ashraf el sayed et al.11
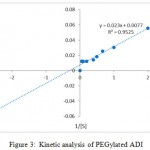 |
Figure 3: Kinetic analysis of PEGylated ADI
|
PEGylation of proteins have been established as a useful technique to overcome the application limitations of protein pharmaceuticals, e.g. decrease in immunogenicity of the enzyme and extended half-life.
The increased durability of PEG-ADI is of great importance for their therapeutic usages, such as drug storage, delivery and activity retention. Previously, random PEGylation reactions were successfully used in most cases of already sanctioned PEGylated products by reacts of PEG molecules with the Ɛ-amino side chains of lysine or the N-terminal α-amino group.
Effect of PH on the Stability of the free and Pegylated ADI
ADI is generally stable and active at neutral and alkaline p H. The effect of p H on the stability of the enzyme was studied in range 4-10. Figure 4 reveals that both the enzymes showed optimal activity 7.5 to 9 with maximum p H 8 for free ADI and 9 for pegylated ADI.
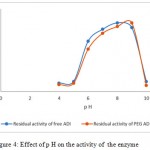 |
Figure 4: Effect of p H on the activity of the enzyme
|
Effect of Temperature on the Activity of Pegylated ADI
The percentages of the activity after 60 minutes of incubation at 37, 45, 50, 60, 70, 80 and 900C are shown in the figure 5. The free and pegylated ADI were active at temperatures from 37 to 800C but they lost their activity at 900C. Both forms retained their maximum activity of more than 85% at 400C, but the process of loss of activity was faster for the free ADI when compared to pegylated ADI when the temperature was increased beyond 500C.As a result immobilized enzyme was more themostable when compared to free ADI.
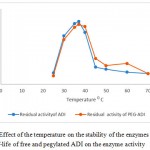 |
Figure 5: Effect of the temperature on the stability of the enzymes In vitro half-life of free and pegylated ADI on the enzyme activity
|
Table 1: In vitro half-life of free and pegylated ADI on the enzyme activity
| Enzyme | T ½ Half values in PBS buffer (days) |
| Non-pegylated ADI | 2.2 |
| Pegylated ADI | 4.2 |
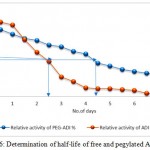 |
Figure 6: Determination of half-life of free and pegylated ADI.
|
Thermal inactivation at 37 °C in human serum as determined through residual activity measurements of non-PEGylated and PEGylated ADI after 1–7 days of incubation (Relative activity = specific activity of ADI or PEG-ADI/ specific activity of ADI , before incubation) × 100%.
In Vitro Anti-Proliferative Activity
The in vitro anti-proliferative activity of the pegylated ADI was assessed against A375-C6, MCF-7, HCT-113 and Jurkat, clone E6-1 cancer cell lines. The results of the assay are given in the figures 7, 8, 9 and 10 respectively. IC50 values for A375-C6, MCF-7, HCT-113 and Jurkat, clone E6-1 cancer cell lines for free ADI were found to be 5.21, 6.3, 8 and 3.13 U/ml whereas that of PEG ADI 11.43, 10.32, 13.46 and 5.21 U/ml. Thus, the anticancer efficacy of the PEG ADI was reduced by 2.19, 1.63, 1.68 and 1.66 respectively, comparing to free enzyme. Same type of results were obtained by A. S. E-Sayed et al.They showed that the anticancer efficiency of the PEG –Arginase was reduced by two and three fold for HEPG-2 and A549, respectively, comparing to free enzyme.12
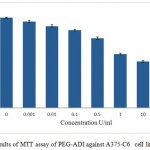 |
Figure 7: Results of MTT assay of PEG-ADI against A375-C6 cell line
|
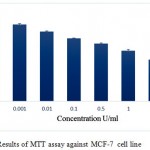 |
Figure 8: Results of MTT assay against MCF-7 cell line
|
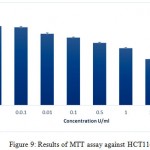 |
Figure 9: Results of MTT assay against HCT116 cell lines
|
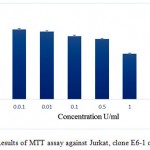 |
Figure 10: Results of MTT assay against Jurkat, clone E6-1 cell line.
|
Conclusion
L-Arginine deiminase of Vibrio alginolyticus 1374 remained effective even after pegylation. Hence it could be a promising candidate for the treatment of various arginine dependent cancers. Further animal studies must be carried out to develop PEG-ADI as a suitable candidate for therapeutic purpose.
Acknowledgments
Authors are thankful to the authorities of Malla Reddy College of Pharmacy for providing the facilities to carry out this work.
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
References
- World Health Organization. 2006- Fact sheet. 297.
- Kumar T. G et al. Biotechnology based drug delivery by pegylation method. International Journal of Research in Ayurveda and Pharmacy. 2011;2(1):95-102.
- Jung-Ki Y et al. Arginine deprivation therapy for malignant melanoma. Clinical Pharmacology: Advances and Applications. 2013:5.
- Vyas et al. Pegylated protein encapsulated multivesicular liposomes: a novel approach for sustain release of interferon alpha. Drug Dev Ind Pharm. 2006;32(6):699-707.
CrossRef - Unissa R., Sudhakar M., Reddy S. K. A. Screening of marine bacterial cultures for extracellular production of L-Arginine deiminases. WJPR. 2015;4(06).
- Unissa R., Sudhakar M., Reddy S. K. A. Condition optimization and production of extracellular l-Arginine deiminase from Vibrio alginolyticus 1374.Current Biotechnology. 2015;4:254-260.
CrossRef - Unissa R., Sudhakar M., Reddy S. K. A. Evaluation of in vitro anti proliferative activity of l-arginine deiminase from novel marine bacterial isolate .BMRJ. 2016;13(5):1-10.
CrossRef - Oginsky E. L. Isolation and determination of arginine and citrulline. Meth Enzymol. 1957;3:639-643.
CrossRef - Lowry O. H., Rosebrough N. N., Farr A. L., Randall R. Y. Protein measurement with the folin phenol reagent. J Biol Chem. 1951; 193:265-275.
- Mosmann T. J Immunol Methods. 1983;65:55-63.
CrossRef - Ashraf S. El-Sayed et al .Arginine Deiminase from Thermophilic Aspergillus fumigatus KJ 434941: Anticancer Activity in vitro Biotechnology Progress.01. 2015;31(2)
- Ashraf S. El-Sayed et al. Purification and immobilization of L-arginase from thermotolerant Penicillium chrysogenum KJ 185377 .1 with unique kinetic properties as thermostable anticancer enzyme. Pharm. Res. 2014:1-10.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





