How to Cite | Publication History | PlumX Article Matrix
Isolation and Characterization of cellulose Producing Bacterial isolate from Rotten grapes
Omchand Singh1, Parmjit S. Panesar1 and Harish K. Chopra2
1Biotechnology Research Laboratory, Department of Food Engineering and Technology, Sant Longowal Institute of Engineering and Technology, Longowal-148106, Punjab, India.
2Department of Chemistry, Sant Longowal Institute of Engineering and Technology, Longowal-148106, Punjab, India.
Corresponding Author E-mail: pspanesarrr@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2455
ABSTRACT: In this present investigation, eleven bacterial cellulose producing isolates were selected out of 34 isolates from different sources. Among the eleven isolates the C18 isolate has been identified as a potential bacterial cellulose producer. Physiological and biochemical tests were carried out to identify the bacteria. The molecular characterization of C18 strain was done by 16 S rDNA analysis and identified as Gluconacetobacter xylinus due to 94% sequence similarity. The maximum bacterial cellulose production (3.96g/L) was obtained after incubation time of 168 h with Hestrin and Hchramm (HS) media in static culture. Structural elucidation of bacterial cellulose was achieved using analytical techniques like FTIR, SEM and X-ray diffraction analysis.
KEYWORDS: Gluconacetobacter xylinus; bacterial cellulose; FTIR; SEM; X-ray diffraction
Download this article as:| Copy the following to cite this article: Singh O, Panesar P. S, Chopra H. K. Isolation and Characterization of cellulose Producing Bacterial isolate from Rotten grapes. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Singh O, Panesar P. S, Chopra H. K. Isolation and Characterization of cellulose Producing Bacterial isolate from Rotten grapes. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=21594 |
Introduction
Cellulose is an extracellular homopolysaccharide naturally synthesized by plant as well as many species of microorganisms. It is the most popular biopolymer in view of its biodegradable and eco- friendly nature. Many bacterial species also synthesize the cellulose that is the alternate source of cellulose production to reduce the demand of plant cellulose.1 Bacterial cellulose is produced by several species of Gram-negative bacteria, such as Acetobacter, Pseudomonas, Salmonella Agrobacterium,Aerobacter, Achromobacter, Azotobacter, Rhizobium, Sarcina, and Salmonella.2,3 Although, these species are well known for the production of cellulose to produce cellulose but Acetobacter xylinum are the most common species for the production of cellulose. These bacteria commonly found in many natural sources such as agro industry waste, fruit waste, flowers, vinegar waste, vegetable waste, Fruit waste, rotten fruits, soil and waste water.4-6 Many researchers showed their interest to isolate the new bacterial cellulose producing strain from these sources which had the potential to produce the large amount of bacterial cellulose. They are Gram negative, rod shape, non pathogenic, aerobic bacteria which synthesized the bacterial cellulose in the form of microfibril and form a thick mat in the interface of fermentation medium.7,8
Bacterial cellulose is free from contaminants such as hemicelluloses, lignin other metabolic products and present in pure form. Bacterial cellulose has several advantage over plant cellulose due to its unique physico-chemical properties such as high purity, high water holding capacity, high crystallinity, hydrophilicity, biocompatibility, high degree of polymerization, high mechanical strength, great elasticity and ultra fine pure nanofibril network. Due to above mentioned distinctive properties, bacterial cellulose is preferred as an alternative of plant cellulose and also to protect the forest deforestation, in addition to its applications in medical science such as wound dressing material, antimicrobial activity, artificial blood vessel and as drug delivery material.9-11 Bacterial cellulose has been used as traditional popular desert “nata de coco” in Philippines and “Kombucha tea” as dietary drink. Bacterial cellulose also used as a coating, binding, thickening and emulsifying agent in food industries.12,13 Keeping in view of the above, the present work was undertaken to isolate and characterize the efficient cellulose producing bacteria from different sources.
Material and methods
Chemicals and Reagents
All chemicals used in present investigation were analytical grade and purchased from Hi-media, Sigma- Aldrich, Ranbaxy and Merck.
Isolation of bacteria
Different samples from (fresh fruit waste, sugar cane waste, vinegar waste, rotten grapes, fruit processing industry waste and fermentation industry waste) were collected for the isolation of bacterial cellulose producing bacteria. One gm of each sample was transferred in modified Hestrin-Schramm14 (HS) medium in 100 ml of flask containing 2.0% D-glucose (w/v), 0.5% peptone (w/v), 0.5% yeast extract (w/v),0.27% Na2HPO4 (w/v), 0.12% citric acid (w/v), 0.2% acetic acid (v/v), 0.5% ethanol (v/v) and 0.01% HS) supplemented with cycloheximide (w/v) to restrict the contamination of fungal and yeast, and incubated at 30°C for 120 to 168 h in static condition. The formation of white pellicle/gelatinous mat on the surface of medium were selected and serially diluted up to 10−7 with 0.9% (w/v) sodium chloride solution. 0.1ml of serially diluted sample was taken and spread on GEY (Glucose, Ethanol and Yeast extract) agar plate containing (2.0% D-glucose, 1.0% yeast extract, 5% ethanol 0.3% CaCO3 and 2% agar) then incubated at 30oC for 48 h or till the colonies were produced. The development of clear zone in the region of the colonies was selected for further fermentation. The thirty four bacterial isolates were isolated from the above mentioned waste and out of thirty four, eleven isolate were selected for further investigations.
Procurement of bacterial Cultures
The standard bacterial cultures Acetobacter aceti MTCC 2623, Acetobacter pasteurianus MTCC 2903, and Acetobacter liquefaciens MTCC 3135 were purchased from Microbial Type Culture Collection Centre, Institute of Microbial Technology, Chandigarh, India.
Identification of Characterization of bacteria
The physiological and biochemical characterization were done as per standard procedure.15,16 The selected cellulose producing isolate namely C18 was examined for 16S rRNA gene sequence analysis as per the standard method by Yukphan et al.17 The 16s rDNA sequencing analysis was done by ABI 3500 Genetic Analyzer and data analysis was also carried out with data analysis software “Seq Scape_ v 5.2’’ (Chromous Biotech, Pvt. Ltd., India). Two universal primer 16s Forward Primer (5’- AGHGTBTGHTCMTGNCTCAS -3’ and 16s Reverse Primer: 5’- TRCGGYTMCCTTGTWHCGACTH -3’) were used. A phylogenic tree was constructed form 1212 bases by the neighbor-joining method 18 by applying MEGA (Molecular Evolutionary Genetics Analysis) programme 19after gene sequences alignments obtained with CLUSTAL W.20 A 16SrRNA, partial gene sequence, similarity matrix between the Gluconacetobacter sp. and isolate C18 was calculated for 1212 bases. The gene sequence was submitted for getting accession number.
Preparation of Fermentation Media and cellulose production from standard cultures and C18 isolate
To investigate the efficiency of isolated strain C18, the Hestrin and Hchramm 14 (HS) medium was prepared with composition glucose (20g/L), yeast extract (5g/L), peptone (5g/L), citric acid (2.7g/L), disodium hydrogen phosphate (1.17g/L). The pH of medium was adjusted to 6.5. The fermentation medium was sterilized at 121°C for 20 minutes. The active culture (2% inoculum) of standard bacterial cultures Acetobacter aceti MTCC 2623, Acetobacter pasteurianus MTCC 2903, Acetobacter liquefaciens MTCC 3135 and isolated Gluconacetobacter xylinus C18 culture were added into 250 ml flasks aseptically and incubated at 30oC for 168 h for cellulose production.
Isolation and Purification of bacterial cellulose
The gelatinous cellulose mat produced during the fermentation was harvested and isolated by centrifugation at 5000 rpm. After centrifugation the biomass washed with distilled or demonized water to removed sugar residues. The washed biomass boiled with 2%, (w/v) NaOH solution for 20 min to remove cells from the cellulose matrix.21 The treated bacterial cellulose neutralized by de-ionized water until the remaining base is removed. The wet and dry weight was measured using method of Yoshino et al.22
Characterization of bacterial cellulose
The surface and structural properties of bacterial cellulose were characterized by fourier transforms infrared (FTIR, model (Bruker Tensor 27), scanning electron microscopy (SEM model, JEOL NEOSCOPE JCB6000), and X-ray diffraction (model, X’Pert3 Powder).
Results and Discussion
Identification and Characterization of C 18 isolate
Eleven bacterial isolates were isolated from different fruits waste for cellulose production on SH (Hestrin & Schramm) medium. C18 isolate was selected for maximum cellulose production among the eleven isolates for further study. The physiological and biochemical characteristics examinations were carried out as per Bergey’s Manual.23 The results revealed that the C18 isolate is the member of Gluconacetobacter sp. as showed in Table 1-2 and Fig.1-3. The bacterium was found to be most similar to Gluconacetobacter xylinus gene for 16SrRNA, partial sequence, strain: NBRC 13693. The next closest homologue was found to be Gluconacetobacter xylinus gene for 16S rRNA, partial sequence, strain: NBRC 16670. The 16S rDNA sequencing confirmed the isolated C18 strain as Gluconacetobacter xylinus (accession number-KY315818)
Table 1: Microscopic characteristics of bacterial isolate C18
| Characteristics | Observation |
| Colour | Creamy |
| Shape | Circular |
| Elevation | Convex |
| Surface | Smooth |
| Margin | Entire |
| Texture | Smooth |
| Shape of cell | Rod shape |
| Diameter of colony | 4mm |
Table 2: Physiological and biochemical characterization of isolated bacterial strain C18
| Biochemical test | Observation/Growth |
| Gram Staining | Negative |
| Methyl Red test | Positive |
| Oxidase test | Positive |
| Indole test | Negative |
| Citrate utilization | Negative |
| Catalase test | Positive |
| Vogues- Proskauer test | Negative |
| ONPG hydrolysis | Negative |
| Urea hydrolysis
Sodium chloride |
Negative
Positive |
| Fermentation test | |
| D-glucose | Positive |
| D- fructose | Positive |
| Lactose | Positive |
| Maltose | Positive |
| Sucrose | Positive |
| Inositol | Positive |
| Erythritol | Positive |
| Xylose | Negative |
| Sorbitol | Positive |
| Starch | Negative |
| pH | |
| 3 | Positive |
| 4 | Positive |
| 5 | Positive |
| 6 | Positive |
| 7 | Positive |
| 8 | Negative |
| 9 | Negative |
| Temperature(°C) | |
| 20 | Positive |
| 25 | Positive |
| 30 | Positive |
| 35 | Positive |
| 40 | Positive |
| 45 | Positive |
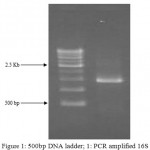 |
Figure 1: 500bp DNA ladder; 1: PCR amplified 16S rDNA of isolated bacteria C18 strain
|
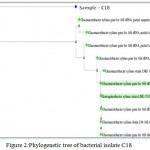 |
Figure 2: Phylogenetic tree of bacterial isolate C18
|
 |
Figure 3: Aligned gene Sequence data of bacterial isolate C18
|
Cellulose production from standard cultures and C18 isolate
The bacterial cellulose production has been depicted in Fig. 4 .The maximum bacterial cellulose (3.96g/L) production was observed by bacterial isolate C18 followed by standard cultures, ie Acetobacter aceti MTCC 2623 (1.78g/L), Acetobacter pasteurinus MTCC 2903 (1.32g/L) and Acetobacter liquefaciens MTCC 3135 (1.18g/L). The produced bacterial cellulose mat is shown in Fig. 5
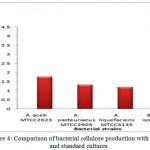 |
Figure 4: Comparison of bacterial cellulose production with isolate and standard cultures
|
 |
Figure 5: Bacterial cellulose produced by isolated Gluconacetobacter xylinus C18 Strain
|
Characterization of bacterial cellulose
The infrared (IR) spectrum shows the specific characteristics absorption bands for bacterial cellulose.24 The infrared spectrum of bacterial cellulose in transmittance mode in the region 4000- 400 cm-1 is given in Fig. 6. The IR spectra depicted the characteristics absorption bands of bacteria cellulose produced by Gloconacetobacter xylinus C18 strain in HS medium. The infrared spectrum showed a strong band in the region of 3344 cm-1 for OH stretching (hydroxyl functional group) and a strong band in the region of 2923 cm-1 due to C-H stretching in line with the already exclusively reports by.9,25 In addition the several typical bands for bacterial cellulose were shown in the region of 1726-1638 cm-1 due to H-O-H bending of water molecules.26 The strong band at 1638 cm-1 shows the presence of carbonyl group (C=O) in bacterial cellulose. The presence of two strong bands due to C-O and C-O-C asymmetric stretching and symmetric stretching were shown in the region of 1163 and 1059-1032 cm-1 respectively, for typical bacterial cellulose.27,28
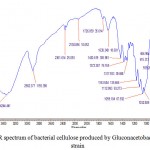 |
Figure 6: FTIR spectrum of bacterial cellulose produced by Gluconacetobacter xylinus C18 strain
|
The scanning electron micrograph of bacterial cellulose produced by Gluconacetobacter xylinus C18 strain in static culture showed the ultrafine microfibril in a well interconnected network structure as given in (Fig.7). The nanosized thread like microfibril were tightly packed and densely woven with each other in line with already reported results by 29 Sarkono et al. Iguchi et al also reported in his study the random assembly of microfibril of less than 100 A° in diameter of freeze dried bacterial cellulose.25
 |
Figure 7: Scanning electron micrograph (SEM) micrograph of bacterial cellulose produced by Gluconacetobacter xylinus C18 strain.
|
X-ray diffraction analysis was used for the determination of crystalline and amorphous contents in the sample.30 The X-ray diffraction profile graph revealed in Fig.8 that the crystallinity peaks of the bacterial cellulose produced by Gluconacetobacter xylinus C18 strain in static culture. The diffraction peaks at 14.1° and 22.5° are indicated the cellulose 1β and 1β. The 1 α and 1β unit cell constituted with one chain and two parallel chains, respectively 31
 |
Figure 8: The X-ray diffraction graph of bacterial cellulose produced by Gluconacetobacter xylinus C18 strain in static culture.
|
Conclusion
From the present investigation it has been concluded that the C18 isolate (isolated from rotten grapefruit) is an efficient producer of bacterial cellulose. The C18 isolate was identified as Gluconacetobacter xylinus as per biochemical and molecular characterization.. The maximum bacterial cellulose (3.96g/L dry wt.) was obtained at temperature 30°C, pH 6.5, inoculum size 2% (v/v) and incubation time 168 h with standard HS medium at static conditions. The characterization of produced bacterial cellulose confirmed using different analytical techniques like FTIR, SEM and X-ray diffractogrph.
References
- Brown Jr R. M. Cellulose structure and biosynthesis: what is in store for the 21st century? Polym. Sci. Part A. Polym. Chem. 2004;42:487-495.
CrossRef - Shoda M., Sugano Y. Recent advances in bacterial cellulose production. Bioprocess. Eng. 2005;10:1-8.
CrossRef - Jung J. Y., Park J. K., Chang H. N. Bacterial cellulose production by Gluconacetobacter hansenii in an agitated culture without living non-cellulose producing cells. Enzyme Microb Technol. 2005;37:347–354.
CrossRef - Toyosaki H., Naritomi T., Seto A., Matsuoka M., Tsuchida T., Yoshinaga F. Screening of bacterial cellulose producing Acetobacter strains suitable for agitated culture. Biosci Biotech Biochem. 1995;59:1498-1502.
CrossRef - Lisdiyanti P., Kawasaki H., Seki T., Yamada Y., Uchimura T., Komagata K. Identification of Acetobacter strains isolated from Indonesian sources, and proposals of Acetobacter syzygii sp.nov. Acetobacter cibinongensis sp. nov., and Acetobacter orientalis sp. nov. J Gen Appl Microbiol. 2001;47:119–131.
CrossRef - Dellaglio F., Cleenwerck I., Felis E., Engelbeen K., Janssens D., Marzotto M. Description of Gluconacetobacter swingsii sp. nov. and Gluconacetobacter rhaeticus sp. nov., isolated from Italian apple fruit. Int J Syst Evol Microbiol. 2005;55:2365-2370.
CrossRef - Ross P., Mayar R., Benziman M. Cellulose biosynthesis and function in bacteria. Rew. 1991;55:35-58.
- McKenna B., Mikkelsen D., Wehr J., Gidley M., Menzies N. Mechanical and structural properties of native and alkali-treated bacterial cellulose produced by Gluconacetobacter xylinus strain ATCC 53524. Cellul. 2009;16:1047-1055.
CrossRef - Klemm D., Schumann D., Udhart U., Marsch S. Bacterial Synthesis Cellulose -Artificial Blood Vessels for Microsurgery. Progress in Scie. 2001;26:1561-1603.
CrossRef - Czaja W., Krystynowicz A., Bielecki S., Brown R. M. Microbial cellulose-The natural power to heal wounds. Biomaterials. 2006;27:145-151.
CrossRef - Ida I. M., Norhayati P., Khairul Z. Bacterial cellulose as secondary metabolite: Production, processing and applications. In: Plant Secondary Metabolites Biological and Therapeutic Significance (Wasim M. W., Prasad K. ed). Apple Academic Press, CRC USA. 2016;1:169-200.
- Jonas R., Farah L. F. Production and application of microbial cellulose. Degrad. Stabil. 1998;59:101-106.
CrossRef - Chawla P. R., Bajaj I. B., Survase S. A., Singhal R. S. Microbial Cellulose: Fermentative Production and application Food Technol. Biotechnol. 2009;47(2):107-124.
- Hestrin S., Schramm M. Synthesis of cellulose by Acetobacter xylinum. II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. J. 1954;58(2):345–352.
- Pure culture techniques in Microbiological Applications Lab Manual in general microbiology 8th edn The McGraw-Hill Companies. 2001;82-88.
- Tortora G. J., Funke B. R and C. L., Case C. L. Microbiology An Introduction, 7th Edn.,Benjamin Cummings Publishing, San Francisco. 2001;69-70.
- Yukphan P., Malimas T., Takahashi M., Potacharoen W., Busabun T., Tanasupawat S., Nakagawa Y., Tanticharoen M., Yamada Y. Re-identification of Gluconobacter strains basedon the restriction analysis of 16S-23S rDNA internal transcribed spacer regions, Gen. Appl. Microbiol. 2004;50:189-195.
CrossRef - Saitou N., Nei M. The neighbor-joining method a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425.
- Tamura K. J., Dudley J., Nei M., Kumar S. MEGA 4 Molecular evolutionary genetics analysis (MEGA) software version 4.0. Biol. Evol. 2007;24:1596-1599.
CrossRef - Thomson J. D., Higgins D. G., Gibson T. J. CLUSTAL W Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673-4680.
CrossRef - Son H. J., Heo M. S., Kim Y. G., Lee S. J. Optimization of fermentation conditions for the production of bacterial cellulose by a newlyisolated Acetobacter sp. A9 in shaking cultures. Biotechnol Appl Biochem. 2001;33:1–5.
CrossRef - Yoshino T., Asakura T., Toda K. Cellulose production by Acetobacter pasteurianus on silicon membrane. Ferment . Bioengineer. 1996;81:32-36.
- Garrity G. M. Bergey’s Manual of Systematic Bacteriology 2ed, Springer, New York. 2002;2:41-43.
- Gunzler H., Gremlich H. U. IR spectroscopy An introduction (Book). 2002.
- Iguchi M.,Yamanaka S., Budhiono A. Bacterial cellulose a masterpiece of nature’s arts. Mat. Sci. 2000;35:261–270.
CrossRef - Rani M. U., Rastogi N. K., Appaiah K. A. A. Statistical optimization of medium composition for bacterial cellulose production by Gluconacetobacter hansenii UAC09 using coffee cherry husk extract – an agro-industry waste. Microbiol. Biotechnol. 2011;21(7):739–745.
CrossRef - Gayathry G., Gopalaswamy G. Production and characterization of microbial cellulosic fiber from Acetobacter xylinum. Indian J. Fiber Res. 2014;39:93-96.
- Jung H. O., Lee O. M., Jeong J. H., Jeon Y. D., Park K. H., Kim H. S., Ann W. G., Son H. Appl. Biochem. Biotechnol. 2010;162:486-497.
CrossRef - Moeljopawiro S., Setiaji B., Sembiring L. Physico- chemical properties of bacterial cellulose produced by newly strain Gluconacetobacter xylinus ANG-29 in static and shaking fermentations. Biosci. Biotech. Res. Asia. 2014;11(3):1259-1265.
CrossRef - Mazumdar S. A standardless method of quantitative ceramic analysis using X-ray powder diffraction. Appl. Crystallogr. 1999;32:381-386.
CrossRef - Nishiyama Y., Langan P., Chanzy H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. Am. Chem. Soc. 2002;124:9074.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





