How to Cite | Publication History | PlumX Article Matrix
Mohammad Javad Kazemi1, Mohammad Kargar2*, Jamileh Nowroozi1, Abbas Akhavan Sepahi1, Abbas Doosti3 and Zahra Manafi4
1Department of Microbiology, Tehran North Branch, Islamic Azad University, Tehran, IR Iran.
2Department of Microbiology, Jahrom Branch, Islamic Azad University, Jahrom, IR Iran.
3Department of Microbiology and Immunology, University of Michigan Medical School, Ann Arbor, Michigan, USA.
4National Iranian Copper Industries Co. Sarcheshmeh Mine, IR Iran.
Corresponding Author E-mail: biolongco@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2417
ABSTRACT: One of the problems with thermoacidophilic microorganisms is purifying them through solid media and that’s why purifying them in most cases has been done using serial dilution. Basal salts and solidification agents used in media have an important role in growing microorganisms. The purpose of this study was preparing the solidified medium with GELRITE and Starch for isolating and purifying indigenous thermoacidophilic microorganisms from Sarcheshmeh Copper Complex in Iran. We used various methods in this study to prepare and optimize GELRITE medium for thermoacidophilic microorganisms. The best method is as follows: GELRITE was separately dissolved in 40 ml of distilled water. The bottle of GELRITE was placed on magnetic stirrer at 90ºC and a magnet was placed in it to stir. The magnet was agitated at 520 rpm for 40 minutes to cause the solution appear dispersed, homogeneous and clear. Various methods were also applied to prepare starch as a solidification factor to culture this group of microorganisms which none succeeded. GELRITE was clear after preparing, and after one month a lot of tiny colonies were observed on GELRITE plates. The first colonies appeared during the first week. When the colonies were removed using a loop -since the colonies were so tiny- the solidified medium was removed, too. Industrializing the thermophilic microorganisms has always encountered difficulties due to culturing insufficiencies and hardness. A confident method for culturing thermoacidophilic microorganisms on a solidified medium with GELRITE and how to keep the plates at the temperature of 70ºC is introduced in this study. This method allows both preparing the solid medium for culturing thermoacidophilic microorganisms and keeping it for a long time (1 month or more) at 70ºC or even higher.
KEYWORDS: GELRITE; Starch; Iran microorganisms; thermoacidophilic
Download this article as:| Copy the following to cite this article: Kazemi M. J, Kargar M, Nowroozi J, Sepahi A. A, Doosti A, Manafi Z. Optimization and Comparison of Different Procedures of Preparation GELRITE and Starch as Solidifying Agents for Culturing Indigenous Thermoacidophilc Microorganisms and How to Keep them at the Temperature of 70 ºc. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Kazemi M. J, Kargar M, Nowroozi J, Sepahi A. A, Doosti A, Manafi Z. Optimization and Comparison of Different Procedures of Preparation GELRITE and Starch as Solidifying Agents for Culturing Indigenous Thermoacidophilc Microorganisms and How to Keep them at the Temperature of 70 ºc. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=17842 |
Introduction
Extreme thermoacidophile is a microorganism which grows at the temperature above 60º C and the pH value lower than 4.0. Most species of studied thermoacidophiles up to now relate to Archaeal orders of Sulfolobales and thermoplasmatals. There are various kinds of thermoacidophiles isolates with aerobic metabolism.1,2 One of the problems with thermoacidophiles is purifying them through solid medium and that’s why purifying them in most cases has been done using serial dilution.3,4 Basal salts and solidification agents used in culture media have an important role in growing microorganisms.5,6 There are various solidification agents such as Agar, Starch, Gelatin, GELRITE, Carrageenan, Isubgol, Guar gum, Xantan gum, etc.7
Agar has been the most common solidification agent for microbial culture media. One of the characteristics of agar as solidification agent is its stability, high clarity, non-toxicity nature and resistance to microbial metabolism during the culturing period.8 But, there were difficulties with the solidification of the autoclaved low pH media, particularly when agar was used as a solidifying agent. An autoclaved medium with pH 3.0 with agar cannot solidify; better outcomes were obtained with GELRITE.9 GELRITE is a heteropolysaccharide made up of glucuronic acid, rhamnose, glucose, and Oacetyl.10 It is anionic and has a high molecular weight and is produced aerobically from the bacterium Auromonas (Pseudomonas) elodea, renamed Sphingomonaspaucimobilis.11,12 This substance is nitrogen free and without phenolic compounds which are toxic for various microbial species.13 GELRITE should be substituted for agar to culture thermoacidophilic microorganisms. Because the prepared plates with GELRITE remain solid in acidic conditions and the temperature above 90º C14. Also, it seems that GELRITE is economically good substitution for agar; because a lesser amount of GELRITE in relation to agar per liter is needed to achieve the same concentration.15 It is purer and clearer than agar and its polymerization takes place in the presence of cations to gel.10 Another important aspect of GELRITE is that allows for easy discernment of various colonies which grow on GELRITE plate. Furthermore, it issuccessfully applied for the separation of many microorganisms unable to grow on agar-containing media.16
Starch is another common solidifying agent which is purer and clearer than agar. A lesser amount per liter is needed to preparea starch-based medium in relation to agar. In cold media, it reversibly absorbs water and swells a little and the swollen process irreversibly occurs in warm water.17 The purpose of this study was preparing the solidified medium with GELRITE and Starch for isolating and purifying indigenous thermoacidophilic microorganisms from Sarcheshmeh Copper Complex in Iran.
Materials and methods
Sampling Sites
The samples were collected from Sarcheshmeh Copper Complex located 160 km southwest of Kerman city in Iran as follows
Five samples of watersrunning in the Mine (in 50 ml bottles) and eight samples of Heapsoil in January 2015. The temperature of the sample area was approximately 6ºC.
Seven samples of waters runningin the Mine (in 50 ml bottles) and ten samples of Heap soil in April 2015. The temperature of the area was around 20ºC and the temperature of the soil in some area of the Heap was 70ºC.
The samples were transported to the laboratory without thermal control and were mixed in a bucket. The pH value was measured by analyzer (Metrohm model 827). ThepH value was2.4.
Microorganism’s enrichment
The first sample was cultured in the autoclaved Acidianusbrierleyi medium which contained the following basal salts (in grams per liter): (NH4)2SO4 (3.00), K2HPO4 x 3 H2O(0.50), MgSO4 x 7 H2O (0.50), KCl (0.10), Ca(NO3)2 (0.01), Yeast extract(0.20), Sulfur (flowers)(10.00).
Cultures were run in a thermostatic rotary agitator (Innova, New Brunswick scientificinnova4200 incubator shaker) at 70°C and were agitated in125 rpm. The pH was adjusted to 1.98 with Merck sulfuric acid. The second sample was cultured in the thermoplasma volcanium medium which contained the following basal salts (in grams per liter):KH2PO4 (3.00), MgSO4 x 7 H2O (1.00), CaCl2x 2H2O (0.25), (NH4)2SO4 (0.20), Yeast extract (1.00), Glucose (5.00). Cultures were run in a thermostatic rotary agitator (Innova, New Brunswick scientific innova 4200 incubator shaker) at 56°C and were agitated in 110 rpm. The pH was adjusted to 2.00 with Merck sulfuric acid.The samples were examined every day regarding the water evaporation. Experiments were performed in 500 ml Erlenmeyer flasks. Each flask contained 150 ml medium and 50 ml mixed samples. The samples were subcultured in the broth medium three times and then were cultured on the solid medium.
The preparation of GELRITE- solidified media from the first sample
The ingredients used in the solid medium are exactly the same ingredients of the broth medium except that Sodium Thiosulfate (10 g. in 1000 ml.) is used instead of sulfur. 0.6% GELRITE (Sigma) was used to prepare the solid medium.
First procedure: dissolve GELRITE in cold distilled waterand stirringstrenuous with hand. All the basal salts, Sodium thiosulfate and GELRITE were dissolved in 90 ml of cold distilled water by stirring strenuously by hand. The pH of the medium was adjusted 1.98 by using Sulfuric Acid (Merck) and then they were autoclaved at 121ºC for 15 minutes. The yeast extract was separately dissolved in 10 ml of distilled water and was autoclaved and then added to the above mixture.
Second procedure: dissolve GELRITE in warm distilled water and stir strenuously by hand. All processes are the same as the first procedure except that all basal saltsin the medium, Sodium thiosulfate and GELRITE were dissolved in 90 ml of warm distilled water by stirring strenuouslyby hand.
Third procedure: dissolve GELRITE with double concentration in cold distilled water and stir with magnetic stirrer. The medium was made similar to those of first and second procedure except that in order to have a strong gel, GELRITE was made as follows:
GELRITE with double concentration + basal salts + Thiosulfate and yeast extract
GELRITE + basal salts+ thiosulfate and yeast extract + 0.1% MgSO4.7H2O
GELRITE+ basal salts+ Thiosulfate and yeast extract + 0.1% CaCl2
Fourth procedure: dissolve GELRITE in cold distilled water and stir with magnetic stirrer. GELRITE was separately dissolved in 40 ml of distilled water. The bottle of GELRITE was placed on a magnetic stirrer at 90ºC and a magnet was placed in it to stir. The magnet was agitated at 520 rpm for 40 minutes to cause the solution appeardispersed, homogeneous and clear. The yeast extract in 10 ml, sodium Thiosulfate in 10 ml and basal saltsof medium in 40 ml of distilled water were separately dissolved in different bottles. The ingredients of these four bottleswere autoclaved separately at 121ºC for 15 minutes and then immediately after autoclaving they were mixed together and poured into a plate at the temperature above 60ºC. The pH of the medium was adjusted at 1.9 by using Merck sulfuric acid on bottle containedbasal salts.
The preparation of starch- solidified media from the second sample
First procedure: dissolve starch solution in cold distilled water and stir with magnetic stirrer. 100 ml of medium was made. 12g of starch solution (C6H10O5)n (Merck) was dissolved in 40 ml of cold distilled water. The yeast extract was dissolved in 10 ml of distilled water and the basal saltsof the medium were dissolved in 40 ml of distilled water and they all were autoclaved separately. Glucose was dissolved in 10 ml of water and was sterilized with a syringe filter. The pH of the medium was adjusted at 2 on bottle containedbasal salts.
Second procedure: dissolve starch solution in warm distilled water and stir with magnetic stirrer. All processes were similar to those of the above mentioned, except that the starch solution was added to the warm water and boiled for 20 minutes.
Third procedure: dissolve starch solution in boiling water. All the processes were done similar to those of the first procedure, except that for each liter one gram starch was prepared. The starch solution was added to the 20 ml of boiling water and boiled for 20 minutes. Afterwards, 50 ml of boiling water from another bottle was added and boiled for another 20 minutes. It was then boiled with a larger amount of boiling water and was cooled later. Finally, fill flask to 1,000 mlwith cooled down boiled water.
Fourth procedure: the use of starch agar. All the processes were performed similar to those of the first method, except that 12.5% starch agar medium was used instead of the starch solution. The starch agar was sterilized using autoclave steam.
Inoculation into the solid medium and incubation
The media -after solidification- were placed in the laboratory temperature for 24 hours and then serial dilution of the last broth subculture was performed and 0.1 ml of 103 dilution was poured on the plate and spread over the medium with an L-shaped rod. Since a high temperature causes the plate to dry rapidly over 2 or 3 days and our purpose is to keep the plate for about one month, the following system was used to maintain the culture media. The media were examined every day to add distilled water to the sponge at the bottom of the container (Figure 1).
 |
Figure 1: The system used to maintain the culture media |
Morphology observation
The morphology of the isolate that was grown on GELRITE observed under a Scanning Electron Microscope PHENOM Pro X (Max Magnification: 45000 X, Resolution: 25 nm, Detectors: BSD, SED and SDD, working voltage: 5 kV, 10 kV & 15 kV).
Results
Preparing and Growth on the solid medium
Four different GELRITE and starch preparation procedures were tested to culture thermoacidophilic microorganism. The first and Second procedures in preparation of GELRITE and starch solution were not successful.Third procedure in both was even not dispersing figure 2.
 |
Figure 2: Unsuccessful procedures in preparation of GELRITE and starch solution. |
The best result for preparingGELRITE and starch was the fourth procedure. GELRITE wasclear after preparing, and after one month a lot of tiny colonies were observedon the plate. The first colonies appeared during the first week.When the colonies were removed using a loop -since the colonies were so tiny- the solidified medium was removed, too figure 3.
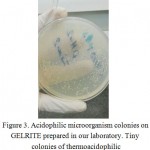 |
Figure 3: Acidophilic microorganism colonies on GELRITE prepared in our laboratory. Tiny colonies of thermoacidophilic microorganism are not shown. |
Morphological observation
The morphological characteristic under Scanning Electron Microscope is shown in figure 4.
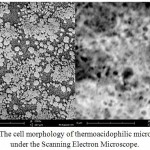 |
Figure 4: The cell morphology of thermoacidophilic microorganism under the Scanning Electron Microscope. |
Discussion
One of the biggest challenges in studying the thermoacidophilic microorganisms is the effect of acidic pH on solidifying capability of solidification agents. Therefore, their pure culturing faces difficulties. Kovačević et al (2013) encountered this problem and they could not manage the problem by autoclaving the medium with 0.2% concentration of GELRITE at the pH value 3.0.9We encountered the same challenge but in the end we succeeded.We tried to prepare and optimize GELRITE medium and provide pure culturing of thermoacidophilic microorganisms which are effective in bioleaching industry.But the following should be taking into consideration for immediate preparation.
Preparing GELRITE requires special care. So, before autoclaving it must be well-viscose and well-dispersed. If preparation is done by hand, it won’t mix well. Mixing process should be done using magnetic stirrer at the temperature above 90º C for about 40 minutes. More importantly, adjusting the pH is of great importance; because if acid is poured on GELRITE, it will prevent solidification. In other study stated that sulfuric acid solidifies the GELRITE medium; our results showed that in no way should sulfuric acid be directly poured on GELRITE to adjust the pH.18 As has been observed around the world, in their experiment for preparing the solid medium base GELRITE at low pH, kept stable the pH by2-(N-morpholino) ethanesulfonic acid (MES) as buffering agent. We didn’t use the buffering agent and our microorganism grew well, because we dissolved GELRITE in a lot of water and our microorganisms grow in range between the pH 1-3, totally.19
Other investigator state that GELRITE should be poured into the plate without delay and at the temperature above 60ºC, also we carried out this procedure.20 But, if a lot of GELRITE is poured into the plate, it may fall off the plate bottom. This is due to placing the plate inverted in the incubator at a high temperature.
There is another important idea in our study: keeping the plate at the temperature of 70ºC. In contrast to Stedman et al beaker filled with water, wet paper towels or cloth also, has no role in preventing the plates from drying14. Due to these reasons, we used the above system.Our result showed that if GELRITE is mixed with basal salts ,MgSO4.7H2O and CaCl2 as strength agents of gel (third method), even if placed over a heater for 3 hours-neither does it solve well nor solidify. It can be inferred that if saltscon centration was inordinate in the medium, they will affect the solidification. Other researches also could not manage this problem by autoclaving. They overcome the problem by sterilizing the medium with microwave oven, but the Ph value of their medium was not lower than 3.09. We couldn’t use the starch solution in this study as a solidification agent. When it was autoclaved, it turned solid inside the autoclave and became useless. Even sterilizing with autoclave steam had no role in preventing its solidification before mixing with the other ingredients of the medium.
Up to now, pure culturing of thermoacidophilic microorganisms has been done by using various sub-cultures on brothmedia. Industrializing the hyperthermophiles has always encountered difficulties due to culturing insufficiencies and hardness. This procedure allows both preparing the solid medium for culturing thermoacidophiles and keeping it for a long time (1 month or more) at 70ºC or even higher.
Acknowledgments
This research was supported by the Sarcheshmeh Copper Complex of Iran. We would like to thank Mr. Ahmad Mogueinejad (Head of Bio Hydrometallurgy Laboratory).
Footnotes
Authors’ Contribution
Mohammad Kargar developed the original idea. Preparation GELRITE and the manuscript wrote by Mohammad Javad Kazemi. Jamileh Nowroozi, Abbas Akhavan Sepahi, Zahra Manafi and Abbas Doosti contributed to the development of the protocol and prepared the manuscript.
References
- Auernik K. S., Cooper C. R., Kelly R. M. Life in hot acid: pathway analyses in extremely thermoacidophilic archaea. Curr. opin. biotecnol. 2008;19(5):445-53.
- Prokofeva M., Miroshnichenko M., Kostrikina N., Chernyh N., Kuznetsov B. Acidilobus aceticus gen. nov., sp. nov., a novel anaerobic thermoacidophilic archaeon from continental hot vents in Kamchatka. Int. J. Syst. Evol. Microbiol. 2000;50(6):2001-8.
CrossRef - Keeling S., Davies K., Palmer M. L., Townsend D., Watkin E. Utilisation of native microbes from a spent chalcocite test heap. Hydrometallurgy. 2006;83(1-4):124-31.
CrossRef - Zeng W. M., Wu C. B., Zhang R. B., Hu P. L., Qiu G. Z. Isolation and identification of moderately thermophilic acidophilic iron-oxidizing bacterium and its bioleaching characterization. Trans. Nonferrous. Met. Soc. China. 2009;19(1):222-7.
CrossRef - Fatima A., Khan S. J. Some factors affecting the in vitro growth of Stevia rebaudiana Bertoni. Iran. J. Plant. Physiol. 2010;1(2):61-8.
CrossRef - Das N., Tripathi N., Basu S., Bose C., Maitra S. Progress in the development of gelling agents for improved culturability of microorganisms. Front. Microbiol. 2015;6:698.
CrossRef - Mateen A., Hussain S., Rehman S. U., Mahmood B., Khan M. A. Suitability of various plant derived gelling agents as agar substitute in microbiological growth media. Afr. J. Biotechnol. 2012;11(45):10362-7.
CrossRef - Babbar S., Jain R., Walia N. Guar gum as a gelling agent for plant tissue culture media. In Vitro. Cell. Dev–Pl. 2005;41(3):258-61.
CrossRef - Kovačević B., Miladinović D., Katanić M., Tomović Z., Pekeč S. The effect of low initial medium pH on in vitro white poplar growth. Glasnik. Šumarskog. fakulteta. 2013;108:67-80.
CrossRef - Silva J. A.T. D. Novel Factors Affecting Shoot Culture of Chrysanthemum (Dendranthema× Grandiflora). Bot. Lith. 2014;20(1):27-40.
CrossRef - Nyonyo T., Shinkai T., Tajima A., Mitsumori M. Effect of media composition, including gelling agents, on isolation of previously uncultured rumen bacteria. Lett. Appl. Microbiol. 2013;56(1):63-70.
CrossRef - Balasubramaniam J., Kant S., Pandit J. K. In vitro and in vivo evaluation of GELRITE® gellan gum-based ocular delivery system for indomethacin. Acta. Pharm. 2003;53(4):251-62.
CrossRef - Dedysh S. N. Cultivating uncultured bacteria from northern wetlands knowledge gained and remaining gaps. Front. Microbiol. 2011;2:184.
CrossRef - Stedman K., Porter M., Dyall-Smith M. The isolation of viruses infecting Archaea. Manual of Aquatic Viral Ecology. 2010;57-64.
CrossRef - Quiala E., Jiménez-Tello M. V., Barbón R., Chávez M., de Feria M. Influence of 6-Benzyladenine and gelling agent on the reduction of hyperhydricity in Tectona grandis L. Rev. Colomb. Biotecnol. 2014;16(1):129-36.
CrossRef - Chen J., Groves R., Zheng Y., Civerolo E. L., Viveros M. Colony morphology of Xylella fastidiosa almond leaf scorch strains. Can. J. Plant. Pathol. 2007;29(3):225-31.
CrossRef - Ubalua A. O., Ihezie C. I., Ikpeama A. I. Cassava Starch: Exploring its potential as an alternative gelling agent for in vitro regeneration and multiplication of sweet potato plantlets. Am. Eurasian. J. Agric. Environ. Sci. 2014;14(8): 748-56.
CrossRef - Smeulders M. J., Pol A., Zandvoort M. H., Jetten M. S., den Camp H. J. O. Diversity and Ecophysiology of New Isolates of Extremely Acidophilic CS2-Converting Acidithiobacillus Strains. Appl. Environ. Microbiol. 2013;79(21):6784-94.
CrossRef - Klerk G. J. D., Hanecakova J., Jásik J. Effect of medium-pH and MES on adventitious root formation from stem disks of apple. Plant cell tissue and organ culture. 2008;95:285-92.
CrossRef - Lin C. C., Casida L. GELRITE as a gelling agent in media for the growth of thermophilic microorganisms. Appl. Environ. Microbiol. 1984;47(2):427-9.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





