How to Cite | Publication History | PlumX Article Matrix
SNP of cGH gene in Egyptian chicken breeds at MspI site
Heba I. Shafey1, Aboelhassan M. D1, E. M. EL-Komy2, Ragaa E. Abd El-karim3 and Karima. F. Mahrous1
1Department of Cell Biology, National Research Center, El Buhouth St., 12311 Dokki, Cairo, Egypt.
2Animal Production Department, Agriculture and Biology Research Division, National Research Centre, El Buhouth St., 12311 Dokki, Cairo, Egypt.
3Animal Production Research Institute, ARC, Ministry of Agriculture, Egypt.
Corresponding Author E-mail: l_fathy@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2414
ABSTRACT: Genetic polymorphism of cGH gene using PCR-RFLP and identification of SNP among different genotypes of Dokki-4, Inshas, El-Salam and Mandarah breeds were studied. Amplification of GH gene (intron 4) for GH2 and GH3 loci produced 563 bp fragment length. The amplified fragments (563 bp) were digested with MspI endonuclease. Loci GH2 and GH3 showed two genotype frequencies TT and TC in all breeds, while genotype CC was absent in Dokki-4 of GH2 and absent in El-Salam of GH3. Analyzed data was compared with Genbank entries of Gallus gallus (KF957977), (KF957978), (KF957979), (KF957980) and (KF957981), the sequences of detected SNP were submitted to GenBank database with the accession numbers KY176737- KY176758. Some Dokki-4 animals showed new two SNPs at positions T77C and C485T. Mandarah and Dokki-4 strains were shared in new three SNPs at positions G130A and C379T which was detected previously at KF957978. Mutation G430A of Mandarah and Dokki-4 was shown in KF957980. Eleven transitions mutations (five A/G and six C/T) and one transversion (G/C) were detected. Six transitions mutations C/T were silent mutations and position G358C was polymorphic in El-Salam.
KEYWORDS: Chicken; cGH; PCR-RFLP; SNP; MspI site
Download this article as:| Copy the following to cite this article: Shafey H. I, Aboelhassan M. D, EL-Komy E. M, El-karim R. E. A, Mahrous K. F. SNP of cGH gene in Egyptian chicken breeds at MspI site. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Shafey H. I, Aboelhassan M. D, EL-Komy E. M, El-karim R. E. A, Mahrous K. F. SNP of cGH gene in Egyptian chicken breeds at MspI site. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=21686 |
Introduction
Chicken is considered as one of the important cheap source of meat and eggs in Egypt. Indigenous breeds are characterized with their disease resistance, adaptation and protection against predators for their plumage (Padhi, 2016). Previously, cross breeding between a native strain and foreign strain was made to produce Egyptian local strains of chicken, followed by selection for different traits on some crosses (Kosba and El-Halim, 2008). Molecular genetics selection is a favorable method to improve the economic important traits in chickens especially meat quality and carcass quality (Zhou et al., 2005). Consequently, health related problems such as obesity, sudden death, immunosuppression and leg problems are occured (Kadlec et al., 2011). Genetic polymorphism that causes genetic diversity can be used to determine the degree of similarities or differences between and within breeds for genetic improvement of farm animals (Jafari et al., 2015).
Circulating growth hormone (GH) is a polypeptide hormone that synthesized and secreted by the pituitary gland and affects the growth traits in broiler production such as growth rate, body weight maturation, metabolism rates, egg production, reproduction, appetite control and aging (Shaw et al., 1991; Feng et al., 1997; Harvey, 2013). Chicken growth hormone (cGH) gene contains 4,101 base pairs having five exons and four introns with large size of introns differing in this consideration from similar mammalian gene (Kansaku et al., 2008; Su et al., 2014). This gene encodes 191 amino acid residues mature growth hormone protein addition to a 25 amino acid signal peptide (Hrabia et al., 2008; Nie et al., 2005).
New approaches such as restriction fragment length polymorphism (RFLP) technique and sequencing of DNA were carried out for detecting the polymorphisms of cGH gene, where previous studies using (RFLP) showed that cGH gene is highly polymorphic in the intron region (Yan et al., 2003; Enayati and Rahimi-Mianji, 2009; Fotouhi et al., 1993). Most of diversities in GH gene are single nucleotide polymorphism (SNP) originating from substitution, deletion or insertion of a single nucleotide (Nie et al., 2005). Studies showed that a single SNP of chicken can influence the performance traits (Huang et al. 1993). Nie et al., 2005 detected T3094C and T3199C mutations in intron 4 of GH gene and MspI-RFLP (the SNP location was based on accession no. AY461843 and associated quantitative trait loci (QTL) with SNPs in domestic animals.
In the present work, RFLP assay and DNA sequence analysis were performed to examine different genotypes and screen the SNP polymorphisms in intron 4 of GH2 and GH3 loci of cGH gene and to investigate their associations with the growth and production traits of the Egyptian Local chicken breed using 84 samples from different four strains from Dokki-4, Inshas, El-Salam and Mandarah.
Material and methods
Total of 122 samples of four local Egyptian chicken strains (Mandarah, Inshas, El-Salam and Dokki-4) were used randomly from each strains of mixed sex as 31 chickens from Mandarah, 34 chickens from Inshas, 28 chickens from El-Salam and 29 chickens from Dokki-4 in order to studying Genetic polymorphism in cGH gene intron 4.
Blood Sampling and DNA Extraction
Blood samples were collected in tubes containing EDTA as anticoagulant and transported to the laboratory under cooled conditions. DNA was extracted and purified from blood samples using the whole blood salting out technique described by Miller et al. (1988). DNA concentration and purity was determined using UV spectrophotometer at optical density of 260 and 280 nm.
Polymerase Chain Reactions (PCR)
PCR amplification was performed using specific primer that was designed on the basis of DNA sequence of the cGH gene accession no. AY461843 (Nie et al., 2005). The designed GH forward primer was GCACTGAGGGACGTGGTTAT and reverse primer was GGCCT CTGGAATCATGGAACC.
Amplification reaction was carried out in a 25 µL volume containing 100 ng genomic DNA, forward and reverse primer of GH gene (both at concentration 10 pmol/µL), 1U Taq polymerase, 2.5 µL Taq polymerase buffer, 200 μM dNTPs. PCR amplification program for cGH gene was performed under cycling conditions of 96°C for 3 min, followed by 35 cycles of 95°C for 15 sec, 60°C for 30-60 sec. and 72°C for 30 sec terminated with elongation at 72°C for 5 min. PCR product was stored at 15°C. The success of amplified PCR product was tested on 2% agarose gel electrophoresis stained with ethidium bromide. The bands were visualized under UV light and the gels were photographed using gel documentation system (Bio-Rad, USA). DNA fragments sizes were estimated by their comparison with 100 bp standard molecular size marker of expected product size of approximately 563 bp.
Restriction Fragment Length Polymorphism (RFLP)
The PCR products were digested using restriction enzyme MspI (Fermentas, Germany) to screen SNPs at T3094C (intron 4, MspI) and C3199T (intron 4, MspI) loci in cGH gene. Ten µL from the PCR products were digested with 5 units of the fast restriction enzyme including specific buffer (Fermentas, Germany) in a final reaction volume of 15 μL. The reaction mixture was incubated at 37 ˚C in water bath for 30 minutes. The obtained restricted fragments were visualized on 2% agarose gel electrophoresis stained with ethidium bromide. The bands were photographed using digital gel documentation system (BioRad, USA). Genotype and allele frequencies were determined using free Lab. Image V2.7 software. It is dispersed free from Proland company (Germany) through the web site: http://www.labimag-ing.com/servlet/ engine/home/ start.html.
Sequencing Analysis and Single Nucleotide Polymorphism
The PCR products, representatives for each detected genotype of cGH gene in different chicken population were purified and sequenced by Macrogen Incorporation (Seoul, Korea). Sequence analysis and alignment of sequence products were carried out using NCBI/BLAST/blastn and BioEdit software in comparison with GenBank Accession numbers to identify single nucleotide substitutions between different detected genotypes.
Results
In the present study, the genetic polymorphisms of intron 4 of cGH gene at two loci GH2 and GH3 were detected in four Egyptian local chicken strains from Dokki-4, Inshas, El-Salam and Mandarah. The single SNPs detection was done by applying two methods, the restriction fragment length polymorphism for the polymerase chain reaction products (PCR-RFLPs) and sequencing of the resulted product.
Genotyping analysis using PCR-RFLP assay
The PCR amplified products of cGH gene of intron 4 for locus GH2 and locus GH3 produced 563 bp fragment length in 122 individuals of four different chicken breeds (Fig. 1a). These PCR amplified fragments (563 bp) were digested with MspI endonuclease (Fig. 1b). Locus GH2 showed three genotypic alleles, which were TT (563 bp), TC (563 bp, 311 bp and 252 bp) and genotype CC (311 bp and 252 bp) in three studied strains Mandarah, Inshas and El-Salam, whereas only two genotypes TT and TC were existed in Dokki-4 chicken strain with the absence of genotype CC. All breeds showed more than 50% genotype frequency for the genotype TT, the highest genotypic frequency (0.84) was recorded for El-Salam strain, while the lowest one (0.52) was detected for Mandarah populations. The TC genotype exhibited higher genotypic frequency comparing with CC genotype in Dokki-4, Inshas and El- Salam (0.35, 0.20 and 0.11) respectively, contrary to Mandarah chicken breed, which possessed higher genotypic frequency for CC genotype (0.28) than TC genotype (0.20) (Table 1). Overall, El-Salam showed the highest allelic frequency of T allele (0.89) and lowest allelic frequency of C allele (0.11).
Table 1: Genotypic and allelic frequencies in four chicken strains Dokki-4, Inshas, El-Salam and Mandarah at locus
| Loci | Breeds | Genotype frequency | Allele Frequncey | |||
| (TT) | (TC) | (CC) | T | C | ||
|
GH2 |
Dokki-4 | 0.65 | 0.35 | 0.00 | 0.83 | 0.17 |
| Inshas | 0.64 | 0.20 | 0.16 | 0.74 | 0.26 | |
| El-Salam | 0.84 | 0.11 | 0.05 | 0.89 | 0.11 | |
| Mandarah | 0.52 | 0.20 | 0.28 | 0.62 | 0.38 | |
| All breeds | 0.66 | 0.21 | 0.13 | 0.76 | 0.24 | |
GH2 of cGH gene digested with MspI.
At locus GH3, the genotypes TT (563 bp), TC (563 bp, 357 bp and 206 bp) and the CC (357 bp and 206 bp) were detected in Dokki-4, Inshas and Mandarah populations. El-Salam chicken breed possessed the two genotypes TT and TC, while genotype CC was disappeared (Table 2). Four studied breeds (Dokki-4, Inshas, El-Salam and Mandarah) showed highest genotype frequency for genotype TT (0.50, 0.44, 0.88 and 0.52), respectively followed by genotype CT that monitored higher genotypic frequency than genotype CC in Dokki-4, Inshas and El-Salam (0.40, 0.28 and 0.11), in contrast to Mandarah chicken breed, the CC genotype demonstrated higher genotypic frequency (0.33) than TC genotype (0.15) (Table 2). El-Salam population showed the highest allelic frequency of T allele (0.94) and lowest allelic frequency of C allele (0.06).
Table 2: Genotypic and allelic frequencies in four chicken strains Dokki-4, Inshas, El-Salam and Mandarah at locus GH3
| Loci | Breeds | Genotype frequency | Allele Frequncey | |||
| (TT) | (TC) | (CC) | T | C | ||
| GH3 | Dokki-4 | 0.50 | 0.40 | 0.10 | 0.70 | 0.30 |
| Inshas | 0.44 | 0.28 | 0.28 | 0.58 | 0.42 | |
| El-Salam | 0.88 | 0.11 | 0.00 | 0.94 | 0.06 | |
| Mandarah | 0.52 | 0.15 | 0.33 | 0.60 | 0.40 | |
| All breeds | 0.57 | 0.25 | 0.18 | 0.70 | 0.30 | |
of cGH gene digested with MspI.
Genotyping Analysis using single Nucleotide Polymorphism (Snps)
MspI–RFLP in intron 4 had T3094C (locus GH2) and C3199T (locus GH3) mutations. The SNP location was based on the published cGH gene sequence GenBank accession no. AY461843. The sequence analysis of the purified and sequenced PCR product of detected two homozygous genotypes TT and CC and heterozygous genotype TC that produced by RFLP-PCR technique for locus GH2 revealed nucleotide substitutions at nucleotide position 3094 corresponding to (252) in our sequences of intron 4 (T/C). Position 206 reflected the homozygous genotype CC, where restriction site of MspI (CCGG) was recognized (Fig. 1c). The graphical figure 2 illustrated two different alleles C and T and overlapping between two alleles C and T and their detailed sequences. The sequence analysis of the purified PCR product for locus GH3 revealed nucleotide substitutions at nucleotide position 3199 on a par with (357) in our results of intron 4 (C/T), two alleles C and T were observed, three genotypes were demonstrated homozygous genotype TT and CC and heterozygous genotype TC. The sequences of detected SNPs of cGH gene in different chicken breeds were submitted to GenBank database with accession numbers
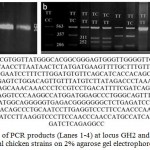 |
Figure 1: a. Detection of PCR products (Lanes 1-4) at locus GH2 and GH3 of cGH gene in local chicken strains on 2% agarose gel electrophoresis.
|
Allele size was about 563 bp. M: 100 bp DNA ladder. b. Three different genotypes obtained after digestion of PCR products of cGH gene with MspI at GH2 and GH3 loci of four studied chicken strains (Dokki-4, Inshas, El-Salam and Mandarah). Lane M: 100 bp ladder marker. Lanes 2, 4, 5, 8, 9, 10 and 11: homozygous genotype TT, Lanes 6, 7 and 13: Heterozygous genotype TC, Lane 12: Homozygous genotype CC at locus GH2. Lanes 5, 6, 7, 10, 11 and 12: homozygous genotype TT, Lanes 4, 9 and 13: heterozygous genotype TC, Lanes 2 and 8: homozygous genotype CC at locus GH3. c. MspI restriction of cGH gene amplified fragments at locus GH2 using Fast PCR C^CGG restriction sites marked in red colour into two fragments 252 bp and 311 bp.
 |
Figure 2: Demonstrations of nucleotide sequence analysis of 563 bp of sequenced results of loci GH2 and GH3 amplified fragment of cGH gene intron 4 and the Genbank submitted partial sequences in chicken strains.
|
The graphical figure of GH2 and GH3 indicates the mutation site of three different genotypes TT, CC and TC in different four studied populations. (a) Described nucleotide position T for allele (TT), (b) Described nucleotide position C for allele (CC) and (c) Described nucleotide SNP for allele (TC) at nucleotide position 3094 and 3199 for GH2 and GH3 loci, respectively.
Sequence Alignment for Cgh Gene Reflecting New Snps
Screening of whole cGH gene intron 4 sequences for four studied chicken strains was examined to look for other SNP polymorphisms (Fig. 3). Our analyzed data was compared with Genbank entries of Gallus gallus Dokki-4 strain GH gene (KF957977), El-Salam strain (KF957978), Hubbard strain (KF957979), Inshas strain (KF957980) and Mandarah strain (KF957981). Comparison of chicken growth hormone sequence with that of five different chicken breeds revealed a total of twelve nucleotide variations including eleven transitions (five A/G and six C/T mutations) and one transversion (G/C mutation). One of the variations at position G358C was polymorphic in El-Salam, whereas C-T mutations were found to be silent mutation without any change in the amino acids.
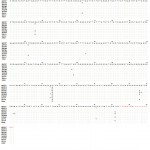 |
Figure 3: Sequencing alignment for cGH gene of four Egyptian chicken strains (Mandarah, El-Salam, two sequences of Dokki-4 and Inshas) in comparison with Genbank entries to describing different new detected SNPs. Forward and reverse primers were represented as the first and last 21 bases.
|
Discussion
Recently, Chicken polymorphic growth hormone gene has vastly studied in various animals. Especially the intron regions that are highly polymorphic and has numerous SNPs, which associated with diverse traits such as meat yield traits, abdominal fat, egg production, linked to body weight and carcass in domestic fowls (Lei et al., 2007; Zhang et al., 2007; Xu et al., 2011). Introns might play a decisive role in the regulation of GH gene expression because of the identification of regulatory elements in intron part of GH gene (Nie et al., 2002). In the present work, SNP was detected in cGH gene and analyzed their association with economic traits in Egyptian four crossing chicken strains, wherever RFLP showed association between these polymorphisms and abdominal fat, egg production, resistance to Marek’s disease or avian leucosis and meat yield traits (Su et al., 2014). Appearance of transition mutation at T3094C and C3199T nucleotides in intron 4, which can be clarified by digestion with MspI restriction enzyme and confirmed by sequencing, are matched with presence of MspI polymorphism in the fourth intron cGH gene of White Plymouth Rock, Poltava Clay, Rhode Island Red, and Borkovskaya Barvistaya chicken breeds (Kulibaba et al., 2015) and eight restriction site that identified in intron 4 of 20 Chinese native chicken populations (Nie et al., 2002). Su et al. (2014) reported that T3094C was associated with egg production traits. Inshas, Mandarah and Dokki-4 are known to be egg type commercial strains that may correlate with T3094C mutation as a result of cross breeding. In contrast to El-Salam strain that is characterized with meat production, moreover there is no evidence that C3199T correlates with meat or egg production.
Alleles T and C can be classified as polymorphic alleles where their allelic frequency less than 1.00 (Nei, 1987), which means that cGH gene in MspI locus was polymorphic. Allele T is predominantly, which is consistent with Makhsous et al. (2013) and Nie et al. (2002), who proved the domination of T allele in Iran and China local chicken populations and resistance allele was dominant for early sexual maturity, while susceptibility allele was dominant for low rate of egg production (Shahnaz et al., 2008). Prevalence of homozygosity against heterozygosity, which was lower than 0.5 in two mutation sites of studied Egyptian chicken might be influenced by some factors such as genetic drift, number of individuals in population, number of Alleles and Alleles frequency (Allendorf and Luikart 2007).
Absence of genotype CC in Dokki-4 at locus GH2 and El-Salam at locus GH3 and increase the frequency of genotype TT in four strains than TC and CC is a good sign for raising breast muscle and decreasing abdominal fat as mentioned by Mu’in and Lumatauw (2013) at the MspI locus. In contrary, two MspI polymorphisms in chicken GH gene have a significant association with abdominal fat rate and breast muscle (Fotouhi et al., 1993; Bingxue et al., 2003). El-Salam strain showed higher genotyping frequency for TT genotype and lower frequencies for TC and CC genotype at both GH2 and GH3 loci of cGH gene that may lead to be preferred strain in meat production. We suggest that GH may regulate breast muscle’s development through a concluded mechanism by (Vasilatos-Younken et al., 2000), who proved that growth hormone regulates the skeleton muscle development by affecting the proliferation and differentiation of muscle cells.
Appearance of new SNPs in intron 4 that is not correlated to MspI sites at El-Salam or Dokki-4 may confirm that they are the most chosen Egyptian strains for high productive and reproductive traits standing on their genotyping frequencies. Beside other discovered SNPs that found in Genbank entries between Dokki-4 and El-Salam and Dokki-4 and Mandarah may also influence meat and egg production and reflect the high polymorphic intron part of GH gene. These SNPs still need more investigations to explore and confirm its relation with commercial required traits in chicken because of identification of functional SNPs of target genes enables direct selection by transmission of specific alleles from parents to offsprings (Khlestkina, 2013) and selecting the genes, which involved in regulation of requisite functions of the organism influencing the intensity of the desirable traits (Kulibaba et al., 2015). For this reason GH could be a genetic locus or linked to a major gene significantly affecting growth and carcass traits in chicken, and the results are also helpful to marker-assisted selection in chickens breeding programs (Bingxue et al., 2003).
In conclusion, Two SNP locations in chicken GH gene at intron 4 were identified in the present study beside other new SNPs. These single nucleotide polymorphisms could serve as useful markers for association studies for growth related traits, since there are indications that there are allelic and genotyping frequency differences among selected Egyptian chicken populations that directed the attention toward El-Salam and Dokki-4 as the most productive strains.
Acknowledgement
This work was supported by the grant of National Research Center (project Code No.9040111)
References
- Padhi M. K. Importance of Indigenous Breeds of Chicken for Rural Economy and Their Improvements for Higher Production Performance. Scientifica. 2016;2016:1-9.
- Kosba M. A and El-Halim H. A. H. A. Evaluation of the Egypt local strains of chicks. Egypt Poult. Sci. 2008;28(IV): 1239-1251.
- Zhou H., Mitchell A. D., McMurtry J. P., Ashwell C. M., Lamont S. Insulin-like growth factor-I gene polymorphism associations with growth, body composition, skeleton integrity and metabolic traits in chickens. Sci. 2005;84:212-219.
CrossRef - Kadlec J., Hosnedlová B., Rehout V., Čítek J., Večerek L., Hanusová L. Insulin-Like Growth Factor-I Gene Polymorphism and its Association with Growth and Slaughter Characteristics in Broiler Chickens. J Agrobiol. 2011; 28(2):157–163.
CrossRef - Jafari A., Pakdel A., Esmailkhanian S. Growth Hormone Gene Polymorphism in Two Iranian Native Fowls (Short Communication). Sci. 2015;3(1):99-104.
- Anthony N., Vasilatos-Younken R., Emmerson D., Nestor K., Bacon W. Pattern of growth and plasma growth hormone secretion in turkeys selected for increased egg production. Sci. 1990;69:2057-2063.
CrossRef - Shaw E. M., Shoffner R. N., Foster D. N., Guise K. S. Mapping of the growth hormone gene by in situ hybridization to chicken chromosome1. Hered. 1991;82:505-508.
CrossRef - Feng X. P., Kuhnlein U., Aggrey S. E., Gavora J. S., Zadworny D. Trait association of genetic markers in the growth hormone and the growth hormone receptor gene in a White Leghorn strain. Sci. 1997;76(12):1770-1775.
CrossRef - Kansaku N., Hiyama G., Sasanami T., Zad_worny D. Prolactin and growth hormone in birds: protein structure gene structure and genetic variation. Poult Sci. 2008;45:1–6.
CrossRef - Su Y. J., Shu J. T., Zhang M., Zhang X. Y., Shan Y. J., Li G. H., Yin J. M., Song W. T., Li H. F., Zhao G. P. Association of chicken growth hormone polymorphisms with egg production. Mol. Res. 2014;13(3):4893-4903.
CrossRef - Hrabia A., Paczoska-Eliasiewicz H. E., Berghman L. R., Harvey S., Rząsa J. Expression and localization of growth hormone and its receptors in the chicken ovary during sexual maturation. Cell Tissue Res. 2008;332:317–328.
CrossRef - Nie Q., Sun B., Zhang D., Luo C., Ishag N. A., Yang G., Zhang X. High diversity of the chicken growth hormone gene and effects on growth and carcass traits. Hered. 2005;96(6):698–703.
CrossRef - Yan B., Deng X., Fei Q., Hu X., Wu C., Li N. Association between single nucleotide polymorphisms of the chicken growth hormone gene and chicken growth and carcass traits. Sci. Bull. 2003;48:1304-1307.
CrossRef - Enayati B., Rahimi G. Genomic growth hormone, growth hormone receptor and transforming growth facor β-3 gene polymorphism in breeder hens of Mazandaran native fowls. Afr J Biotechnol. 2009;8(14):3154-3159.
- Fotouhi N., Karatzas C. N., Kuhnlein U., Zadworny D. Identification of growth hormone DNA polymorphisms which respond to divergent selection for abdominal fat content in chickens. Appl. Genet. 1993;85:931-936.
CrossRef - Huang N., Cogburn L. A., Agarwal S. K., Marks H. L., Burnside J. Over expression of a truncated growth hormone receptor in the sex-linked dwarf chicken: evidence for a splice mutation. Mol Endocrino. 1993;l 7:1391–1398.
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracüng DNA from human nucleated cells. Nucleic Acids Res. 1988;16:12-15.
CrossRef - Lei M., Luo C., Peng X., Fang M., Nie Q., Zhang D., Yang G., Zhang X. Polymorphism of growth-correlated genes associated with fatness and muscle fiber traits in chickens. Sci. 2007;86:835-842.
CrossRef - Zhang X. L., Jiang X., Liu Y. P., Du H. R., Zhu Q. Identification of Avai polymorphisms in the third intron of GH gene and their associations with abdominal fat in chickens. Sci. 2007;86(6):1079-1083.
CrossRef - Xu H. P., Zeng H., Zhang D. X., Jia X. L., Luo C. L., Fang M. X., Nie Q. H., Zhang X. Q. Polymorphisms associated with egg number at 300 days of age in chickens. Mol. Res. 2011;10:2279-2289.
CrossRef - Nie Q., Ip S. C. Y., Zang X., Leun F. C., Yang G. New variations in intron 4 of growth hormone gene in Chinese native chickens. Hered. 2002;93(4):277–279.
CrossRef - Kulibaba R. A., Yurko P. S., Liashenko Y. V. MspI-Polymorphism in Fourth Intron of the Growth Hormone Gene in Chicken Populations of Different Breeds: Analysis of the Causes of Additional Restriction Pattern Origin. Cytol Genet. 2015;49(6):372–377.
CrossRef - Nei M. Molecular Evolutionary Genetics. Columbia University Press, New York. 1987.
- Makhsous S., Mirhoseini S., Zamiri M and Niazi A. Polymorphisms of growth hormone gene in a native chicken populatio association with egg production.Vet. Inst. Pulawy. 2013;57(1):73–77.
CrossRef - Shahnaz S., Shadma F., Rank D. N., Khanna K., Joshi C. G. Growth hormone gene polymorphism and its correlation with different traits in Bantam and Leghorn chicken. Indian Poult. Sci. 2008;43(2):123–127.
Cross Ref - Allendorf F. W., Luikart G. Conservation and the Genetics of Populations 1st edn. Blackwell Publishing Oxford, UK, 2007;118.
- Mu’in M., Lumatauw S. Identification of MspI Polymorphism in the forth intron of chicken growth hormone gene and their associations with growth traits in Indonesia native chickens. Anim. Prod. 2013;15(1):1-7.
- Bingxue Y., Xuemei D., Jing F., Xiaoxiang H., Changxin W., Ning L. Single nucleotide polymorphism analysis in chicken growth hormone gene and its associations with growth and carcass traits. Sci. Bull. 2003;48(15):1561-1564.
CrossRef - Vasilatos-Younken R., Zhou Y., Wang X., McMurtry J., Rosebrough R., Decuypere E., Buys N., Darras V., Van der Geyten S., Tomas F. Altered chicken thyroid hormone metabolism with chronic GH enhancement in vivo: consequences for skeletal muscle growth. Endocrinol. 2000;166:609-620.
CrossRef - Khlestkina E. K. Molecular markers in genetic studies and breeding. J. Genet. Appl. Res. 2013;4(3):236–244.
Cross Ref

This work is licensed under a Creative Commons Attribution 4.0 International License.





