How to Cite | Publication History | PlumX Article Matrix
Nizam Uddin Farooqui and C. B. S. Dangi
Department of Biotechnology, R. K. D. F. University, Gandhinagar Bhopal (M.P) India 462033.
DOI : http://dx.doi.org/10.13005/bbra/2431
ABSTRACT: Taxonomic diversity and morphological diversity are interrelated. The diverse mangrove systems can grow in wide range of geographical, climatic, soil and hydrological conditions.The main aim of this work is to record different morphological features ( such as size of stem, leaves, flowers etc. in different species of Mangroves found in deferent ecological niches ( like swamps, fresh water bodies, salt water, plainsand mountains etc).
KEYWORDS: Geographical; Morphological; Mangrove; Plainsand
Download this article as:| Copy the following to cite this article: Farooqui N. U. Dangi C. B. S. Taxonomic Diversity of Mangroves: Analysis of Morphological Characteristics in Different Ecological Niches. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Farooqui N. U. Dangi C. B. S. Taxonomic Diversity of Mangroves: Analysis of Morphological Characteristics in Different Ecological Niches. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=22901 |
Introduction
The term “mangrove” was considered as chief component in Portuguese term called “mangue” and the English term called “grove.” The terms in French language were called as “manglier” and “paletuvier”.1 In Spanish the term is coined as “manglar”. In case of Dutch the term “vloedbosschen” describes the mangrovian community and the term “mangrove” refers the individual trees. Usage in German follows English. In Surinam, “mangro” refers Rhizophora.2 It was believed that most of these terms had their origin from the Malaysian word called as “manggi-manggi” which means “above the soil.” At present, the above mentioned term lost its usage in Malaysia but the term is addressed in the eastern part of the Indonesia to refer the Avicennia species.
Mangroves were quite old and it might have possibly arised after the evolution of first angiosperms before 114 million years.3 Rhizophora and Avicennia belong to the first genera to evolve at the end of the Cretaceous period.4 Records of Pollen had provided vital facts about the subsequent frequency in radiation. Fossil studies on sediments in China and Leizhou Peninsula suggest that the mangroves were expanded their range from south to north. After reaching a limit on the northern region of the delta in Changjiang by the mid-Holocene, a similar case study was conducted from the latent regions of Holocene. The samples found in Bermuda suggest the fact that the mangroves were established before 3000 years and the level of sea rise were gradually reduced from 26 cm to 7 cm per century.5
Dr. Spalding gave a rough estimate of 18 million in hectares, with 41.4% in the south and southeast Asia along with an additional range of 23.5% in Indonesia. Mangroves were restricted to latitudes between 30°N and 30°S. Extension in north for this limit occurs in Bermuda (32°20’N) and Japan (31°22’N).In case of extension in south, Australia (38°45’S) and New Zealand (38°03’S) were highlighted.
Mangroves were distributed within their ranges and they were strongly affected by temperature6 and moisture.7 Currents in large-scale may also influence the level of distribution by the process of preventing propagates from reaching certain low areas.8 Individual mangrove species differ in length along with their establishment in tolerance and growth rate. These factors were also consistent around the world for producing a distributional range for most of the species, as illustrated in Table.1.
Table 1: Taxonomy and global distribution of Avicennia
| Species | Distribution |
| Avicennia alba Blume | Southeast Asia, South Asia, East Asia & Archipeligo. |
| Moldenke ex Molodenke and Avicennia balanophora Stapf | Australia |
| Avicennia bicolor Standley | Central / South America |
| Avicennia eucalyptifolia (Zipp. ex Miq.) Moldenke | Australia |
| Avicennia germinans (L.) Stearn | Southeast USA & Central/South
America |
| Avicennia lanata Ridley | Malay Archipeligo |
| Avicennia marina (Forsk.). Vierh. | Malay Archipeligo, Southeast Asia, Southwest Pacific, Africa, South Asia, Australia & East Asia |
| Avicennia officinalis L. | Southeast Asia, Malay Archipeligo, East Asia & Australia. |
| Leechman ex Moldenke and Avicennia schaueriana Stapf | Central / South America |
| Avicennia africana Palisot de Beauvois | Africa |
Taxonomy
Tomlinson (1986) had categorized mangroves as Major, Minor and associates. Major species were the strict mangroves and they were recognized by most of the following features like (1) Their occurrence in mangal. (2) Their role in community in forming pure stands. (3) They have aerial roots to perform the mechanism for gas exchange. (4) Their physiological mechanisms to exclude & excrete salt. (5) Their viviparous structure for reproduction and (6) Species isolation from their terrestrial relatives with respect to taxonomy.
The minor species of mangroves seldom form pure stands. As per the report of Tomlinson (1986), the kingdom of mangroves includes 34 Species for 9 Generalization in 5 Families and the minor can contribute to an additional of 20 species9-11 in 11 Families and 11 Genera for a total of 54 species in mangrove for 20 Genera with 16 Families.
In 1992, Duke had identified species (69) of mangrove that belong to Genera (26) for Families (20) and One member among the family falls in the division of Fern (Polypodiophyta) and the remainders were present in the Magnoliophyta (angiosperms). Families that contain only mangroves belong to Pellicieraceae, Nypaceae, Avicenniaceae, and Aegialitidaceae. The Two orders (Myrtales & Rhizophorales) contain 25% of family members in Mangrove.After the process of reconciling the common features from experts like Tomlinson (1986) and Duke (1992), it was estimated that mangrove species (65) were found in Genera (22) of Families (16).
The problems associated with mangrove taxonomy were based on the nature of hybridization between the species. For instance, the difference between the R. stylosa in Australia and Rhizophora mucronata in the eastern Africa were still unclear. Rhizophora lamarckii were found in New Caledonia. Rhizophora x annamalayana was found in the mangrove forest of south India.12-16 Initially it was identified as R. lamarckii and then it has been re identified as a new hybrid between R. apiculata and R. mucronata. Some hybrids like Rhizophora x harrissoni were not been confirmed by the principle of wax chemistry. Molecular analysis may help us to resolve the problems associated with taxonomy. For example, the data were obtained from the DNA sequence of rbcL (chloroplast gene) indicate the fact that the Rhizophoraceae belongs to Myrtales family that includes the families Humiriaceae, Malphighiaceae and Euphorbiaceae.
Ecological Condition
Salt Regulation
Mangroves were tolerant to high level of salt content and have a mechanism for obtaining fresh water due to strong potential of osmosis with respect to sediments. The species avoid the load of salt by a combination of excretion and accumulation. For example, Bruguiera, Ceriops and Rhizophora possess ultra filters in root systems to exclude salt when extracting water from soil. There are other genus (For e.g., Aegiceras, Acanthus and Avicennia) to take the content of salt and excrete it through the glands specialized for salts in their leaves.
Species which excretes the salt allows the accumulation of salt into xylem other than the non-excretors and it still excludes about 90% of salinity. Excretion of salt is always an active process and it was evidenced on the activity of ATPase in plasma lemma of excretory cells.24 Regulation of the process was taken care by the hypodermal cells present in leaf to store water and salt.
Excoecaria and Lumnitzera species accumulate salts in their leaf vacuoles to become succulent. The Concentrations of salt can be reduced by the process of storing them in the bark or the wood or transferring them into senescent leaves. As the saline content of water increases, some species had become more conservative in the usage of water and hence it has achieved greater tolerance.25 In case of south Florida, Rhizophora mangle decreases the stress of salt by the utilization of water present in surface as its primary source of water. With respect to wet season, the biomass of fine root increase in response to the decrease in salinity of the surface water and thus an enhanced uptake was observed with respect to low-salinity of water.
Most of the species in mangrove regulates their salt directly. However, they may also synthesize and accumulate solutes other than salts for regulating their osmotic balance. For example, Aegialitis annulata, Aegiceras corniculatum and Laguncularia racemosa can accumulate proline and mannitol. Avicennia marina accumulate glycine betaine, asparagine and stachyose. Sonneratia alba synthesizes the purine base of nucleotides and it helps the species to adapt a salt load of 100 mM of NaCl. In order to facilitate water flow from root to leaves, the potential of water at leaves were held lower (-2.5 to -6 MPa) than roots (-2.5 MPa).
Mangroves conserve water and regulate the internal concentrations of salt. Slow water uptake and low transpiration and were not the character of all species in mangrove. High transpiration rates in Rhizophora apiculata and Avicennia alba and were measured by Becker et al.. In case of Bruguiera cylindrical the transpiration rates vary with season and the change is corresponded to the movement in stomata and the oscillatory behavior of stomata in Avicennia germinans were affected by factors that trigger a change in hydraulic flow throughout plant. This includes an increase in the deficit of osmotic potential and vapor pressure of the substrata. In 1997, the effects of salinity on sugar catabolism with respect to the leaves and roots of Avicennia marina were studied by Fukushima et. al. and it was observed that the pathway was significantly affected by salinity.
Ecological Condition Photosynthesis
Mangroves were characteristic with respect to the photosynthesis of C3+. Basak et al. (1996) found a significant variation with respect to inter- and intraspecific aspects in photosynthetic activity of 14 species in mangrove and it suggest that the rate of photosynthesis might have a base in genetics. This possibility was continuously supported on the basis of observations with respect to rate of photosynthesis of Bruguiera. In contrast, researchers in other fields have also shown that the rate of photosynthesis in certain species and it was affected strongly by the environmental conditions and for example, the conditions favoring lower content of salinity can reduce the loss of carbon in Avicennia germinans and Aegialitis annulata. Fluctuating soil salinities can lead to a significant decrease in lowering the intercellular level of CO2 concentration and reducing the level of photosynthesis in the scrub forests of south Florida.
The stunted mangroves have lower canopies than mangroves in fringe forests which experiences less variability in salinity. Steinkem and Naidoo had demonstrated the fact that the temperature can affect the photosynthetic rate of Avicennia marina and it has an influence with respect to the overall rate in growth.
Strong sunlight can play a vital role in reducing photosynthesis through the process of inhibition in mangrove. The photosynthetic rates can get saturated at a relatively low level, despite of their presence in the tropical environments with high sunlight.26 The lower rate of photosynthesis can also be associated to the concentration of pigment present in the leaves of zeaxanthin. In order to prevent the damage of photo system, the mangroves converts the excess light energy by the xanthophyll cycle for the conversion of Oxygen to peroxides and phenolics.
Kathiresan et al had nicely demonstrated the fact that the applications of aliphatic alcohol may have a stimulatory effect on the photosynthesis of mangrove. Treatment with alchohal like triacontanol (a long-chain aliphatic alcohol) had widely increased the photosynthetic rate of Rhizophora apiculata by 25%. A similar treatment with methanol (a short-chain aliphatic alcohol) had increased photosynthesis of R. mucronata by 61%.
Materials and Methods
A survey has been conducted recently to study the mangrove vegetation in the areas with great diversity with confirmed morphology. The study was designed to analyze the differences between morphology and anatomy of few mangroves species with respect to their adaptation to saline habitat. Leaves, stems, roots from different families of mangrove were collected from those areas during 2011 and they were placed in a polythene bag containing water. Thin sections and cross sections were cut by using a sharp razor blade. The sections were immersed in water to avoid the formation of air bubbles. Sections were stained with Safranine. Excess of stain were washed with water. Then, the selected saplings were stained with fast green solution and the excess of were washed with water. Thereafter, sections were mounted in Glycerol and covered with cover slip and observed under microscope.
Results and Discussion
Mangroves had evolved and they had flourished their dynamic setting. Collectively mangroves were specialized their morphology and physiology. These attributes contribute for the limited variability of the various species. The distributional range for every species in mangrove had reflected their action to the influencing factors that are dominant at the region in local & global scales. Mangroves inhabit the tropical areas of the world and their positioning in latitudes is generally examined by 20°C in the winter and the isotherm of the respective hemisphere (Fig. 1). Exceptions correspond to the path of currents in ocean for circulation where the distributions of mangrove are in broader on the margins in eastern range of continental regions and they were constrained in the western region. Distributional patterns in current day depends on the specialized propagates of water-buoyant nature in mangroves. The dispersal is affected by the coverage of water & the land in continental areas. The major barriers that restrict the dispersal of marine organisms in coastal areas (mangroves included) across the globe, namely: (1) Africa and Euro-Asia (2) North and South America; and the oceans of (3) the North and South Atlantic; (4) the eastern Pacific. The relative effectiveness differs for each of these barriers, depending on dispersal ability, evolutionary appearance and geological history of the respective species.
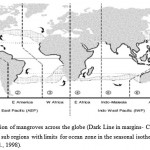 |
Figure 1: Distribution of mangroves across the globe (Dark Line in margins- Coastal areas) show global regions and sub regions with limits for ocean zone in the seasonal isotherm i.e. 20°C (source: Duke et al., 1998). |
 |
Figure 2
|
Conclusion
There is a need for wide propaganda to promote understanding and knowledge by analyzing the observations in detail with an appropriate data. The key lies in studies by subjecting one or other approaches, namely:
Assessment in chemical, genetic and morphological variations among the related taxa for developing an evolutionary understanding among the individual taxa across the entire range of distribution;
Comprehensive compilations in the distributional records with respect to the revised assessments in the genetic and morphological characteristics of the extant groups of related taxa (For e.g. multi-specific genera) across their range of distribution;
A review regarding the synthesis of records about fossil to identify the gap in space and time to demonstrate continuity between the taxa of fossil and extant. In addition, questions were raised about global distributions and genetic discrepancy still remains unanswered. There were solid reasons for the many gaps but still there are no answers for the fundamental questions with respect to the early dispersal and evolution in modern communities among mangroves.
Challenges remain pertinent in the systematics and botanical taxonomy, where the extant of mangrovian taxa is still incomplete. This aspect continues to be somewhat surprising by considering the fact that, there are only 70-80 taxa of mangroves in the world. Furthermore, those unresolved taxonomic questions can still be applied equally to restrict the rare taxa to identify the common ones. In addition, our understanding of relationships within and among mangroves were not initiated or assisted by the development of recent advancements in molecular techniques. Our limited progress is due to lack of coordination between laboratory analyses and field observations regarding the comparison of morphological and genetic and data for analysis.
References
- Allen J. A. Mangroves as alien species the case of Hawaii. Global Ecology and Biogeography Letters. 1998;7:61-71.
CrossRef - Baker J., Furnas M., Johnson A., Moss A., Pearson R., Rayment G., Reichelt R., Roth C and Shaw R. A Report on the Study of Land-Sourced Pollutants and Their Impacts on Water Quality In and Adjacent to the Great Barrier Reef. Department of Primary Industries, Brisbane. 2003.
- Bargagli R. Trace Elements in Terrestrial Plants: An Ecophysiological Approach to Biomonitoring and Biorecovery. Springer, Berlin. 1998.
- Bell A. M and Duke N. C. Effects of Photosystem II-Inhibiting herbicides on mangroves– preliminary toxicology trials. Marine Pollution Bulletin 2005;51:297-307.
CrossRef - Blasco F. Climatic factors and the biology of mangrove plants. 1984;18-35.
- Snedaker C and Snedaker J. G., eds. The mangrove ecosystem: research methods.UNESCO, Paris.
- Boto K. G and Wellington J. T. Soil characteristics and nutrient status in a northern Australian mangrove forest. Estuaries. 1984;7:61-69.
CrossRef - Briggs J. C. Biogeography and plate tectonics. Elsevier, Amsterdam. 1987.
- Cavanagh J. E., Burns K. A., Brunskill G. J., Coventry R. J. Organochloride pesticide residues in soils and sediments of the Herbert and Burdekin river regions, North Queensland– implications for contamination of the Great Barrier Reef. Marine Pollution Bulletin. 1999;39:367-375.
CrossRef - Chapman V. J., ed. Wet coastal ecosystems. Elsevier Scientific Publications Co., Amsterdam, New York, Oxford. 1977.
- Duke N. C. Mangrove Floristics and Biogeography. 1992;63-100.
CrossRef - Duke N. C. Genetic diversity, distributional barriers and rafting continents – more thoughts on the evolution of mangroves. Hydrobiologia. 1995;295:167-181.
CrossRef - Duke N. C. Australia’s Mangroves. The authoritative guide to Australia’s mangrove. 2006.
- The University of Queensland & Norman C Duke. 200.
- Duke N. C and Bunt J. S. The genus Rhizophora (Rhizophoraceae) in northeastern.
Australian Journal of Botany. 1979;27:657-678.
CrossRef - Duke N. C and Wolanski E. Muddy coastal waters and depleted mangrove coastlines – depleted seagrass and coral reefs. 2001;77-91.
- Duke N. C and Watkinson A. J. Chlorophyll-deficient propagules of Avicennia. marina and apparent longer term deterioration of mangrove fitness in oil-polluted. Marine Pollution Bulletin. 2002;44:1269-1276.
CrossRef - Duke N. C and Burns K. A. Fate and effects of oil and dispersed oil on mangrove ecosystems in Australia. Environmental implications of offshore oil and gas development in Australia: further research. A compilation of three scientific marine studies. Australian Petroleum Production and Exploration Association APPEA, Canberra. 2003;232-363.
- Duke N. C., Ball M. C and Ellison J. C. Factors influencing biodiversity and distributional gradients in mangroves. Global Ecology and Biogeography Letters. 1998;7:27-47.
CrossRef - Duke N. C., Benzie J. A. H., Goodall J. A and Ballment E. R. Genetic structure and evolution of species of the mangrove genus Avicennia (Avicenniaceae) in the Indo-West Pacific. Evolution. 1998;52(6):1612-1626.
- Duke N. C.,Burns K. A., Swannell R. P. J., Dalhaus O and Rupp R. J. 2000.
CrossRef - Dispersant use and a bioremediation strategy as alternate means of reducing the impact of large oil spills on mangrove biota in Australia: the Gladstone field trials.Marine Pollution Bulletin. 41:403-412.

This work is licensed under a Creative Commons Attribution 4.0 International License.





