How to Cite | Publication History | PlumX Article Matrix
Kajaria Divya1, Tripathi J. S1, Tiwari. S. K.1 and Pandey. B. L2
1Faculty of Ayurveda, Department of Kayachikitsa, IMS, BHU, Varanasi, India.
2Department of Pharmacology, IMS, BHU, Varanasi, India.
Corresponding Author E-mail: divyakajaria@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2480
ABSTRACT: This study was aimed at evaluating the safety profile, acute and subchronic toxicity of Shirishadi polyherbal ayurvedic compound in rodents, commonly used in the treatment of Bronchial Asthma. Acute toxicity was evaluated in wister albino rats by administering orally graded doses of extract of Albezzia lebbeck, Cyperus rotandus and Solanum xanthocarpum (Shirishadi Compound) in the dose of 2000, 5000 mg/kg, 10, and 20 g/kg body weight of animal and observed continuously for first 4 h and hourly for next 12 h, then 8 hourly for next 56 h (72 h, acute toxicity study). The study had been strictly followed the OECD guidance. Adult Wister albino rats were divided into 3 groups with 5 animals in each group and a control group, fed with doses of 5, 100 mg and 150 mg/ml once in a day for 30 days. Effect of drug was evaluated on hematological parameter after every 7 days and histological study was done after sacrificing the animals on 31st day. The median acute toxicity value (LD50) of the extract was found to be infinite and drug was found to be totally non toxic as after ingestion of 20 g no animal found to be dead. Hematological profile shows no abnormal elevation in level of Serum-Bilirubin, GOT, GPT, urea and creatinine. There was no any evidence of toxicity in histopathological studies.
KEYWORDS: Asthma; Histopathological study; LD 50 value;Polyherbal compound
Download this article as:| Copy the following to cite this article: Divya K, Tripathi J. S, Tiwari S. K, Pandey B. L. An Evaluation of Acute Toxicity and Subchronic Toxicity of Hydroethanolic Extract of “Shirishadi”- A Polyherbal Ayurvedic Compound in Rodents. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Divya K, Tripathi J. S, Tiwari S. K, Pandey B. L. An Evaluation of Acute Toxicity and Subchronic Toxicity of Hydroethanolic Extract of “Shirishadi”- A Polyherbal Ayurvedic Compound in Rodents. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=24498 |
Introduction
Bronchial asthma is a chronic inflammatory disease of airways. It may be allergic or nonallergic in origin. Sustain inflammation leads to remodeling of airways and cause diminution of their proper functioning.
Asthma is said to be only prevented and not cure and this fact underlies its pathophysiological distinctiveness. Asthma proves to be lethal in its acute manifestation as acute onset of severe bronchoconstriction may cause grave respiratory distress due to lack of proper oxygen saturation. Asthma whether acute or chronic always associated with permanent damage of normal architecture of airways and thus need a careful and proper management. Although contemporary medicines proves beneficial and life saving in bronchial asthma but ultimate dependence on corticosteroids and wide range of toxic side effects forces researcher to think in different dimension and search for some alternative remedy that prove beneficial in preventing as well as cure asthma.
In an attempt to search some cheap, effective, and fast acting remedy of bronchial asthma, some ayurvedic compound are prepare and planned to be given in aerosol form through nebulization. Preclinical study of aforementioned said research trial consists of toxicity study of ayurvedic compound in animal model. The route of administration of drug selected consists of both oral and Inhalation route. Whenever an investigator administers a chemical substance to a biological system, different types of interactions can occur and a series of dose-related responses result. In most cases these responses are desired and useful, but there are a number of other effects which are not advantageous. These may or may not be harmful to the patients. The types of toxicity tests which are routinely performed bypharmaceutical manufacturers in the investigation of a new drug involve acute, sub-acute and chronic toxicity. Acute toxicity is involved in estimation of LD50 (the dose which has proved to be lethal (causing death) to 50% of the tested group of animals). Determination of acute oral toxicity is usually an initial screening step in the assessment and evaluation of the toxic characteristics of all compounds. This article reviews the methods so far utilized for the determination of median lethal dose (LD50) and the new changes which could be made. This has to go through the entire process of validation with different categories of substances before its final acceptance by regulatory bodies. The following toxicity studies were performed in the present research trial:
Acute toxicity study
Sub chronic toxicity study
Acute Lethality (Barlow et al., 2002; Bürger et al., 2005; Bruce, 1985)
Objectives
To determine Maximum Tolerated Dose (MTD) and No Observable Effect Level (NOEL).
To determine the Median Lethal Dose (LD50) after a single dose administered through oral route which is the intended route of administration in humans.
To identify potential target organs for toxicity, determine reversibility of toxicity, and identify parameters for clinical monitoring.
To help select doses for repeated-dose toxicity tests.
Duration
Mortality within 72 hours was cut-off time for acute toxicity however survival of animals were observed up to 2 weeks following single dose administration.
Parameters
(Combes, 2004)
Mortality.
General clinical observation of behavior.
Weight change.
Gross autopsic examination of dying animals.
Experimental Animal
(Crook, 2006 Daswani et al., 2006)
Adult Charles Foster Albino rats (70 + 30g) of either sex were obtained from the Animal Research Branch of the Institute of Medical Sciences, Banaras Hindu University, Varanasi . The animals were housed in polyvinyl cages and were fed on commercial pellet diet (Amrut, Pranav Agro Industries Ltd, India). They were grouped & housed under standard conditions of temperature (22 ± 2C0), relative humidity (60 ± 5%) and 12:12 light/dark cycle, where lights on at 0700 and off at 1900 h). The saline fed group served as control and one group was treated with a standard drug in each protocol. Before experimentation, the animals were kept on fast for 24 h but water was given ad libitum except during experimental test period. During experiments, animals were also observed for any alteration in their general behavior.
All the experiments and the care of the laboratory animals were according to current ethical guidelines by the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India, New Delhi.
The research protocol was approved by Institutional Research committee of Institute of Medical Sciences, BHU- India.
Plant Material and Extraction
TheplantsAlbezzia lebbeck,Cyprus rotandus & Solanum xanthocarpum, were collected from local market of Varanasi. The identification of the drugs was done by Prof.A.K. Singh, Department of Dravyaguna, S.S.U., Varanasi ( Identification number DG/ AKS / 604). Hydroalcoholic Extraction ( Distilled water: Ethanol = 2:1) of drugs was carried out by hot percolation method through soxhlet appratus. Thereafter extracts were dried using rotatory evaporator and dried extracts were put to the process of standradization.
Drug Treatment
Standradized extract of Ayurvedic compounds dissolved in distilled water was orally administered as graded doses of 50mg, 100mg, & 150mg/ 100g of bwt of animal once daily for 30 days for Subchronic toxicity study. Control rats were treated with equal volume of normal saline. The test substance is administered in a single dose by gavage using a stomach tube or a suitable intubation canula. For Acute toxicity study, standradization extract ofShirishadi Polyherbal Ayurvedic compounds was administer as escalated doses of 200mg, 500mg, 1g & 2g/ 100g bwt to know the LD 50 value. At each step (i.e. for each dose, starting from lowerone) three rodents were used as per OECD guideline (Guideline No.425).
Method Employed
(W.J. Dixon,1965 , R.D. Bruce,1985 & R.D. Combes, 2004 )
OECD guidelines number 425was followed for Acute Oral Toxicity study with Up-and –Down- Procedure (UDP) .The concept of the up-and-down testing approach was first described by Dixon and Mood
The method is easiest to apply to materials that produce death within one or two days. According to guideline minimum number of animal should be used for the experiment at each step. The test is divided into two parts: Limit test & Main test.
Limit Dose at 2000mg /Kg
Step Ist
One Albino rat of group A weighting was administered 200mg ofShirishadi Hydroalcoholic extract dissolved in 1ml of distilled water orally through intubation cannula.
Step IInd
The rats were observed for 24 hour and when no toxic side effects appears, four more albino rats were taken in group A and administered 2000mg / Kg body weight of Shirishadi extract. The animals were watch for 48 hours. After 48 hours all the rats were survived with no toxic side effect.
Table 1: Body weight variation of treated rats with various doses of the Hydroethanolic extract of Shirishadi during Acute Toxicity Study.
|
Groups |
Body weight of rats (Before Treatment) | Dose of Drug administered
|
Dose of Drug/ 100g body weight | Body weight of Rats (After treatment) | ||
| After 24hrs | After 48hrs | After 72 hrs | ||||
|
A
|
Ist – 90g | 0.9ml |
200mg/ ml |
110g | 110g | 110g |
| Iind – 70g | 0.7ml | 95g | 100g | 105g | ||
| IIIrd – 65g | 0.65ml | 85g | 87g | 100g | ||
|
B
|
Ist – 70g | 0.7ml |
500mg/ml |
90g | 90g | 95g |
| IInd – 70g | 0.7ml | 75g | 90g | 95g | ||
| IIIrd – 50 g | 0.5ml | 60g | 62g | 65g | ||
|
C
|
Ist – 95g | 0.95ml |
1g/ml |
115g | 115g | 120g |
| Iind – 75g | 0.75ml | 95g | 97g | 100g | ||
| IIIrd – 75g | 0.75ml | 100g | 100g | 100g | ||
|
D |
Ist – 70g | 0.75ml+ 0.75ml | 1g/ml twice within 1/2hrs interval to make total of 2g. | 85g | 90g | 90g |
| Iind – 75g | 0.75ml + 0.75ml | 90g | 90g | 90g | ||
| IIIrd – 65g | 0.65ml + 0.65ml | 65g | 65g |
65g |
||
Limit Test at 5000 mg/kg
Step Ist
One albino rat of Group B 100gm waas treated with 5000mg/ Kg of Shirishadi extract dissolved in distilled water and observed for 48 hrs.The ratsurvived and found healthy after 48 hrs of treatment.
Step IInd
When no observable toxic sign and symptoms appears in the albino rats treated with 5000mg/Kg of polyherbal extracts, two more albino rats in group B were added and administered polyherbal drugs in the dose of 5000mg/Kg. The rats were observed for 48 hours.
As no sign and symptom of toxicity appeared in limit test, 12 more albino rats were taken, divided into two groups and selected for main test.
Next escalated dose of extract (Shirishadi) was decided to be 10gm/Kg body weight administered in albino rats of Group C. The rats were observed for 48 hrs and when no sing and symptom of toxicity appears six more albino rats were taken and divided into two groups namely D and were given next escalated dose of 20gm/ Kg body weight in two divided dose at the interval of 30 minutes.
As even after administration of trial drug in the dose of 20gm/ Kg body weight, no rat die, LD50 of present trial drug is found to be infinite.
To confirm that the present trial drug is non toxic OECD guidline, Page No. 40, was followed. According to the guideline if 2gms oral administration of drug dose not kill the rodent it should be considered as non –toxic.
Table 2: Effect of Hydroethanolic extract of Shirishadi Extract on various organ weights after 3days of acute toxicity study.
|
Dose/ 100gm of animals |
Lung/ 100g bwt
N= 3 |
Liver/ 100g bwt
N= 3 |
Stomach/ 100g bwt
N=3 |
Kidney /100 g bwt.
N= 3 |
Heart/ 100g bwt
N=3 |
Adrenal gland/ 100g bwt
N= 3 |
Testis/ 100g bwt.
N=3 |
Spleen/ 100g bwt N=3 |
| Control | 948+ 1.56 | 3200+ 0.08 | 1078+ 1.78 | 350+ 2.05 | 330+ 1.98 | 7.1+ 3.67 | 884+ 0.56 | 281+ 1.05 |
| 200mg | 875+ 2.34 | 3600+ 1.23 | 1136+2.3 | 340+1.56 | 320+ 2.08 | 7.8+ 0.68 | 865+ 1.78 | 286+ 0.56 |
| 500mg | 890+ 0.54 | 3450+ 1.54 | 1010+ 0.01 | 300+ 0.04 | 305+ 3.56 | 6.9+ 2.36 | 840+ 0.47 | 261+ 1.23 |
| 1gm | 930+ 0.90 | 3545+ 3.21 | 1080+ 2.78 | 365+ 0.64 | 315+ 0.01 | 7.5+ 1.67 | 884+ 0.02 | 290+ 0.56 |
| 2gm | 950+ 1.04 | 3711+ 0.21 | 1178+ 0.08 | 370+ 1.02 | 327+ 1.36 | 8.0+ 2.98 | 870+ 0.05 | 280+ 0.01 |
All the values are Mean +SDE , where n=3.
Table 3: Acute Toxicity study of Hydroethanolic Etract of Shirishadi in rodents;Death after 7days of treatment.
| Group | No. of Albino Rats | Dose of Extract | No. of Dead rats | % Cumulative
dead of rats |
| A | 3 | 200mg/ 100g bwt | 0.00 | 0% |
| B | 3 | 500mg/100g bwt | 0.00 | 0% |
| C | 3 | 1g/100g bwt | 0.00 | 0% |
| D | 3 | 2g/ 100g bwt | 0.00 | 0% |
Sub- Chronic Toxicity Study
(R.D.Combes,2004 & S.M.Barlow,2002)
Objectives
To establish a “no observable effect level” (NOEL).
To characterize dose-response relationships following repeated doses.
To identify and characterize specific organs affected after repeated administration.
To predict a reasonable and appropriate dose for chronic exposure studies (maximum tolerated dose or MTD).
Duration
30 days.
Test System/Animal System
Rodent
Dose Administration
Male and female Wistar albino rats weighing 100 ± 20 g were maintained on standard animal feeds and provided with water ad libitum. The animals were weighed and divided into seven groups of five animals each. After overnight fasting of the rats, the control group received a dose of 0.5 ml of normal saline solution orally once a day for 30 days. The treated groups respectively received the following doses: 50, 100, 150 mg/kg body weight of the hydroalcoholic extracts (Shirishadi &Bharangyadi) orally once daily for 30 days (Pieme et al., 2006; Joshi et al., 2007; Mythilypriya el al., 2007).The animals were then weighed every five days, from the start of the treatment, to note any weight variation.
Parameters
Mortality
Weight change
Signs of toxicity
Clinical pathology
Pathology and histopathology
Table 4: Variation in body weight of normal and treated rats with various doses of the Shirishadi hydroethanolic extract during 30 days of subchronic toxicity study.
| Dose/Kg bwt | Day 1
Bwt(gms) |
Day 5
Bwt(gms) |
Day 10
Bwt(mgs) |
Day 15
Bwt(mgs) |
Day 20
Bwt(mgs) |
Day 25
Bwt(mgs) |
Day 30 Bwt(mgs) |
|
Control |
100 | 101 | 105 | 110 | 112 | 113 | 115 |
| 105 | 106 | 108 | 110 | 113 | 115 | 118 | |
| 110 | 112 | 114 | 116 | 118 | 120 | 122 | |
| 102 | 103 | 105 | 108 | 110 | 111 | 112 | |
| 100 | 100 | 105 | 106 | 110 | 110 | 112 | |
|
50mg
|
115 | 120 | 122 | 125 | 128 | 130 | 134 |
| 100 | 105 | 108 | 115 | 120 | 122 | 126 | |
| 110 | 115 | 120 | 125 | 130 | 132 | 135 | |
| 105 | 108 | 112 | 115 | 118 | 122 | 125 | |
| 110 | 115 | 118 | 120 | 125 | 128 | 132 | |
|
100mg |
110 | 112 | 115 | 120 | 125 | 130 | 132 |
| 105 | 110 | 118 | 120 | 122 | 125 | 128 | |
| 100 | 105 | 108 | 110 | 112 | 115 | 118 | |
| 115 | 120 | 125 | 128 | 130 | 132 | 135 | |
| 120 | 124 | 130 | 132 | 135 | 140 | 142 | |
|
150mg |
100 | 102 | 105 | 108 | 110 | 112 | 115 |
| 115 | 115 | 117 | 119 | 122 | 125 | 128 | |
| 120 | 122 | 124 | 128 | 130 | 133 | 135 | |
| 122 | 125 | 126 | 130 | 132 | 135 | 138 | |
| 108 | 110 | 112 | 115 | 118 | 120 | 122 |
Table 5: Body weight variation of normal and treated rats with various doses of the extract during 30 days of subchronic toxicity study.
| Dose/ Kg bwt | Day 1 | Day 5 | Day 10 | Day 15 | Day 20 | Day 25 | Day 30 |
| Control
50mg 100mg 150mg |
103+ 1.88
108+ 2.54 110+ 3.53 113+4.04 |
104+2.16
112+ 2.69 114+3.44 114+4.14 |
107+1.74
116 +2.607 119+ 3.83 116+ 3.86 |
110+1.67
120+ 2.36 122+3.79 120+4.08 |
112+ 1.46
124+ 2.28 126+3.89 122+4.02 |
113+1.77
126+2.05 128+ 3.86 125+4.23 |
115+ 1.98
130+2.06 132+ 3.70 127+4.20 |
All the values are Mean +SDE , where n=5.
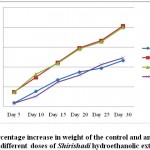 |
Figure 1: Percentage increase in weight of the control and animals treated withdifferent doses of Shirishadi hydroethanolic extract
|
Table 6 : Effect of Shirishadi Compound on Biochemical profile
|
Parameter |
After 15 days | After 30 days | ||||||
| Control | 50mg/
Kgbwt |
100mg/
Kgbwt |
150mg/
Kbwt |
Control | 50mg/
Kgbwt |
100mg/
Kgbwt |
150mg/ Kbwt |
|
| B.Urea | 30.2 ± 2.78 | 36 ± 5.86 | 33.6± 2.73 | 44.6± 4.82 | 46.7± 4.87 | 39.2 ± 4.89 | 30.4± 1.43 | 50.6± 6.54 |
| S.Creatinine | 0.5± 0.05 | 0.5 ± 0.45 | 0.6± 0.09 | 0.5± 0.02 | 0.68± 0.09 | 0.52 ± 0.02 | 0.5 ± 0.05 | 0.68± 0.08 |
| S.Bilirubin (Total) | 0.6± 0.78 | 0.5± 0.45 | 0.74± 0.08 | 0.78± 0.12 | 0.5± 0.02 | 0.68± 0.04 | 0.5± 0.02 | 0.70± 0.12 |
| S.Bilirubin(Direct) | 0.5 ±0.54 | 0.5 ± 0.05 | 0.42 ± 0.06 | 0.56 ± 0.20 | 0.52± 0.03 | 0.48 ± 0.09 | 0.5± 0.08 | 0.5± 0.05 |
| SGOT (AST) | 150± 4.57 | 100 ± 1.25 | 143± 7.40 | 163.7 ± 14.12 | 150.8± 3.35 | 123.3± 9.40 | 107.1± 8.67 | 168.8± 5.89 |
| SGPT (ALT) | 60± 4.38 | 73.6 ± 2.56 | 43.4± 5.41 | 68± 1.90 | 40.3± 5.36 | 65.48± 5.29 | 69.4± 10.63 | 70± 4.35 |
All the values are Mean +SDE , where n=5.
Table 7: Sub-Chronic Toxicity study in albino rats with Shirishadi extract
| Groups | No. of Rats | Dose of extract | No.of Dead Rats | % Cumulative dead of Rats |
| A | 5 | Control | 0 | 0% |
| B | 5 | 50mg/ Kgbwt | 0 | 0% |
| C | 5 | 100mg/Kgbwt | 1 | 20% |
| D | 5 | 150mg/ Kgbwt | 2 | 40% |
Results and Discussion
The result shows that Shirishadi polyherbal compound is innocuous and very safe for therapeutic use (Yashada, 2006). There were no abnormalities detected in histopathological study nor any
biochemical abnormalities were identified in organ sample of sacrificed rodents after acute toxicity study. In chronic treatment with higher doses, death rate showed 20 and 40% cumulative increase. However there is no evidence of organic toxicity in the animals. Studies may be performed in very exclusive conditions to evaluate the issue of long term safety. The acute toxicity study of the extract (Shirishadi compound) indicated no changes in the behaviour and in the sensory nervous system responses in the animals. Also no adverse gastrointestinal effects were observed in the albino rats. The LD50 calculated for the drug is found to be infinite (according to OECD guidelines) as no rat died even after oral administration of dose 20 g/kg bwt (Table 1). Histopathalogical study of viscera’s showed no microscopic or macroscopic abnormality without any change in colour, no congestion, no necrosis or any other sign of toxicity (Plates 6, 7, 8, 9 and 10).
 |
Figure 1a: Showing different groups of animal |
 |
Figure 2. Measuring diet for animals during for toxicity studies sub- chronic toxicity study |
 |
Figure 3: Showing animals taking diet during toxicity studies |
 |
Figure 4: Different concentration of drugs |
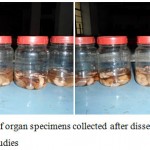 |
Figure 5: Samples of organ specimens collected after dissection for histopathological studies |
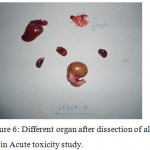 |
Figure 6: Different organ after dissection of albino rats in Acute toxicity study. |
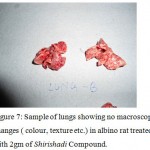 |
Figure 7: Sample of lungs showing no macroscopic changes ( colour, texture etc.) in albino rat treated with 2gm of Shirishadi Compound.
|
Biochemical profile including –Liver function test (LFT), Renal function test (RFT) show no abnormal change substantiated that drug has no renal or hepatotoxicity (Table 2). Percentage cumulative dead after acute toxicity study was found to be 0% (Table 3).
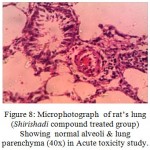 |
Figure 8: Microphotograph of rat’s lung (Shirishadi compound treated group) Showing normal alveoli & lung parenchyma (40x) in Acute toxicity study.
|
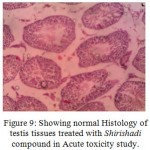 |
Figure 9: Showing normal Histology of testis tissues treated with Shirishadi compound in Acute toxicity study.
|
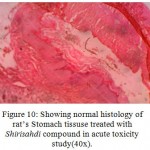 |
Figure 10: Showing normal histology of rat’s Stomach tissuse treated with Shirisahdi compound in acute toxicity study(40x).
|
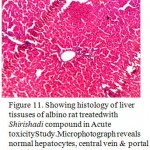 |
Figure 11. Showing histology of liver tissuses of albino rat treatedwith Shirishadi compound in Acute toxicityStudy.Microphotograph reveals normal hepatocytes, central vein & portal tract (40x).
|
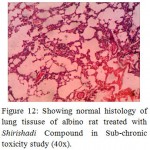 |
Figure 12: Showing normal histology of lung tissuse of albino rat treated with Shirishadi Compound in Sub-chronic toxicity study (40x).
|
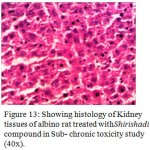 |
Figure 13: Showing histology of Kidney tissues of albino rat treated withShirishadi compound in Sub- chronic toxicity study (40x).
|
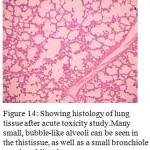 |
Figure 14: Showing histology of lung tissue after acute toxicity study.Many small, bubble-like alveoli can be seen in the thistissue, as well as a small bronchiole running obliquely
|
Body weight of albino rat’s increases propionate with dose of drug but become stabilized at higher doses (Figure 1). As there was increase intake of feed with increasing dose of drug, the increase in body weight was indicative of anabolic effect (Tables 4 and 5). Biochemical analysis showed no toxic or abnormal changes suggesting that drug is safe for long duration internal use (Table 6). There was 40% cumulative death in group treated with 150 mg of Shirishadi extract during subchornic study whereas 20% (Table 7) in group treated with 100 mg of extract.
As there were no morbid signs found prior to death and in addition to this there was no any evidence of toxicity in histopathological studies (Plates 11, 12, 13 and 14) excludes the possibility of death due to toxic effect of drug.
References
- Barlow S. M., Greig J. B., Bridges J. W. Hazard identification by methods of animal-based toxicology. Food Chem. Toxicol. 2002;40:145-191.
CrossRef - Bruce R. D. An Up-and-Down Procedure for Acute Toxicity Testing. Fund. Appl. Tox. 1985;5:151-157.
CrossRef - Bürger C., Fischer D. R., Cordenunzzi D. A., de Borba A. P. B., Filho V. C., dos Santos A. R. S. Acute and subacute toxicity of the hydroalcoholic extract from Wedelia paludosa (Acmela brasilinsis)(Asteraceae) in mice. J. Pharm. Sci. (www.cspsCanada.org). 2005;8(2):370-373.
- Combes R. D., Gaunt I., Balls M. A Scientific and Animal Welfare Assessment of the OECD Health Effects Test Guidelines for the Safety Testing of. 2004.
- Chemicals under the European Union reach System. ATLA. 32:163-208.
- Crook M. A. Clinical Chemistry and Metabolic Medicine.7th Edition. Hodder Arnold, London. 2006;426.
- Daswani G. P., Brijesh S., Birdi J. T. Preclinical testing of medicinal plants: advantages and approaches. 2006.
- Dixon W. J. Staircase Bioassay: The Up-and-Down Method. Neurosci. Biobehav. Rev. 1991;15:47-50.
CrossRef - Dixon W. J. The Up-and-Down Method for Small Samples. J. Amer. Statist. Assoc. 1965;60:967-978.
CrossRef - Ekaidem I. S., Akpanabiatu M. I., Uboh F. E., Eka O. U. Vitamin B12 supplementation: effects on some biochemical and haematological indices of rats on phenytoin administration. Biokemistri. 2006;18(1):31- 37.
- Farnsworth R. N. Review on biological and phytochemical screening of plants. J. Pharm. Sci. 1966;55:225-276.
CrossRef - Ghosh M. N. Fundamentals of experimental pharmacology, 2nd Edition. Scientific Book Agency, Calcutta. 1984;154-157.
- Joshi C. S., Priya E. S., Venkataraman S. Acute and subacute studies on the polyherbal antidiabetic formulation Diakyur in experimental animal model. J. Health Sci. 2007;53(2):245-249.
CrossRef - Klaasen C. D., Amdur M. O., Doull J.Casarett and Doull’s Toxicology: The basic science of poison. 8th Edition. Mc Graw Hill, USA. 1995;13-33.
- Mythilypriya R., Shanthi P., Sachdanandam P. Oral acute and subacute toxicity studies with Kalpaamruthaa, a modified indigenous preparation on rats. J. Health Sci. 2007;53(4):351-358.
CrossRef - Pieme C. A., Penlap V. N., Nkegoum B., Taziebou C. L., Tekwu E. M., Etoa F. X., Ngongang J. Evaluation of acute and subacute toxicities of aqueous ethanolic extract of leaves of (L) Roxb (Ceasalpiniaceae).African J Biotech. 2006;5(3):283-289.
- Shah A. M. A., Garg S. K., Garg K. M. Subacute toxicity studies on Pendimethalin in rats. Indian J. Pharmacol. 1997;29:322-324.
- Tilkian M., Sarko C. B. M., Tilkian G. A. Clinical implication of laboratory tests. 2nd Ed. The C.V Mosby Company, St Louis, Missouri, USA. 1979.
- Wasan K. M., Najafi S., Wong J., Kwong M. Assessing plasma lipid levels, body weight, and hepatic and renal toxicity following chronic oral administration of a water soluble phytostanol compound FM-VP4, to gerbils. J. Pharm. Sci. 2001;4(3):228-234. (www. ualberta.ca/csps).
- Yash wantrao Chavan Academy of Development Administration (YASHADA)Workshop proceedings on approaches towards evaluation of medicinal plants prior to clinical trial. Organized by the foundation for medical research at Pune, India. 2006;60-77.

This work is licensed under a Creative Commons Attribution 4.0 International License.





