How to Cite | Publication History | PlumX Article Matrix
Biological Synthesis of Gold Nanowires by pseudomonas Aeruginosa
Roya Rashidi and Hamid Reza Ghorbani
Department of Chemical Engineering, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran.
Corresponding Author E-mail: hamidghorbani6@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2513
ABSTRACT: The present and fast-growing demands of the synthesis of nanoparticles with various areas suchas medicine and energy, catalysis, electronics and chemistry increased day to day. In the pastdecade, nanoparticles are prepared from wet chemical method, where chemicals used are quietflammable and often toxic. In this report, we are going to synthesize gold nanowires from bacterium Pseudomonas aeruginosa. Gold nanowires were synthesized through the reduction of aqueous Au3+ ion using the growth culture supernatants. Gold nanowires were formed within 1 h of gold ion coming in contact with the cell filtrate. The synthesized gold nanowires were characterized from TEM, UV-Vis and DLS.An absorption peak at 550 nm in Uv–Vis spectrophotometer was detected indicating the presence of gold nanowires. The DLS analysis showed gold nanowires with size of 90±15 nm.
KEYWORDS: Gold; Nanowires; Pseudomonas aeruginosa
Download this article as:| Copy the following to cite this article: Rashidi R, Ghorbani H. R. Biological Synthesis of Gold Nanowires by pseudomonas Aeruginosa. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Rashidi R, Ghorbani H. R. Biological Synthesis of Gold Nanowires by pseudomonas Aeruginosa. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=24448 |
Introduction
In modern sciences, the field of nanotechnology is one of the most active area of research whichsuddenly bring brigh through amongs all sciences. Then field of nanoparticles exhabitscompletely a new and improved properties based which comes with totally new characteristicssuch as morphology and distribution and size. Here the question arises why we reduce the sizeof a material and why we are interested to study small size materials.1,2 The excellent biocompatibility of GNPs is due to the ease ofconjugation to various biomolecules, i.e. peptides, proteins,enzymes, aptamers and antibodies.3 Biomolecules secretedby bacteria are involved in the synthesis of GNPs, and they play arole as capping agents absorbed on the surfaces of GNPs.4 GNPs have many biomedical applications, such as detectorsof disease biomarkers, immunosensors, diagnostic imagingagents, drug or gene delivery vectors, and side-directed biosensorsin the photothermal destruction of tumors.5,6 Moreover,in biomedical applications of GNPs, their physicochemical andoptical properties with regard to surface plasmon light absorptionand scattering are important; these properties depend on theGNP’s size and shape.7,8 Thus, providing for the controlledbiosynthesis of GNPs of proper size and shape is of greatimportance for such applications.For synthesis of gold NPs, a number of synthesis approaches are available for examplephotochemical and chemical reactions,4 gold NPs thermal decomposition,5 reduction insolution,6 electrochemical,7 sonochemical, 8 microwave assisted process9 and recentlyvia green synthesis.10 gold NPs attracted much attention in in the field of medicine and biology due to its fascinatingphysicochemical properties. It has been known that gold product has bacterial and stronginhibitory effects as well as broad spectrum of antimicrobial activity which is used to treat andprevent many diseases notably infections.11 However, the reported gold NPs possessantiflammatory 12 and antifungal.13,14
However, the biological synthesis of gold nanowires using microorganism has been seldom reported. This study demonstrated the extracellular synthesis ofstable goldnanowires.
Experimental
The mixedculture of Pseudomonas aeruginosawas cultured in a medium containingpyruvate, yeast extract, NaCl, NH4Cl, and K2HPO4 at pH 7 and30 °C. After 72 h of fermentation, the cells were separated fromthe culture broth by centrifugation (8000 rpm) at 15 °C for 30min and washed three times with deionized water to obtain about 1 g wet weight of cells.The harvested cells werethen resuspended in 10 mL of deionized water for 15 days. Thecells were then removed by centrifugation, and the aqueoussupernatant obtained was cell-free extract (CFE).The CFE solution thusprepared was a light yellow liquid and was used for the reductionof HAuCl4. To test tubes containing 10 mL of CFE solutionwas added 50-100 mL of 0.001 M aqueous HAuCl4 solution.All experiments were conducted at 30 °C and pH 6 for 48 h,during which time reduction of Au3+in all of the reactionmixtures had occurred. The reduction of the Au3+ions in the solutions was monitored by sampling the aqueous component and measuring the UV–Vis spectrum of solutions. Particle-size distributions of the samples were also obtained using dynamic light scattering (DLS). Furthermore, the gold nanoparticles were characterized by transmission electron microscopy (TEM).
Results and Discussions
One of the useful techniques for structural characterization and stability of the gold NPs in the aqueous solution is UV-Vis spectroscopy.It is well known that gold can be reduced from Au3+to Au0 by a cell-free extract (CFE) of Pseudomonas aeruginosa.The appearances of the peaks indicate the characteristics of surface plasmon resonance of nano gold particles. Fig (1) indicates the UV-Vis spectra recorded from aqueous solution of gold ions. The absorption band at about 550 nm is known to be due to surface plasmon resonance in nano-gold solutions.
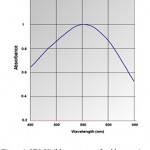 |
Figure 1: UV–Visible spectrum of gold nanowires
|
Dynamic light scatteringis a used method for the determination of nanoparticle size.The gold particles’ size histograms show that the nanoparticles size is 90±15 nm (Fig. 2). The shape and morphology of the synthesized gold nanowires were elucidated with the help of transmission electron microscopy (TEM) further confirming the formation of gold nanowires(Fig. 3).
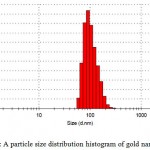 |
Figure 2: A particle size distribution histogram of gold nanowires
|
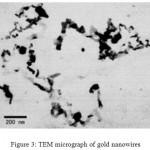 |
Figure 3: TEM micrograph of gold nanowires
|
Conclusions
In the field of nanotechnology, an urgent need to the development of ecofriendly and reliableprocess for the synthesis of metallic nanoparticles. We demonstrated that, the low cost, naturalbiological reduction of pseudomonas aeruginosa produce metal nanostructures with an efficientgreen protocol which avoids toxic and hazardous waste and solvents presence. This study showed an economical, simple and rapidroute to synthesize goldnanowires.
References
- Guo Y., Song G.,Wang R. W. Murray. Does core size matter in the kinetics of ligand exchanges of monolayer-protected Au clusters. J Am Chem Soc. 2005;127:2752-2757.
CrossRef - Huang J., Zhang J., Chen C. H. Free radical scavenging efficiency of Nano-Se in vitro. Free Radic Biol Med. 2003;35:805–813.
CrossRef - Durán., Marcato P. D., De Souza G., Alves O., Esposito E. Antibacterial Effect of Silver Nanoparticles Produced by Fungal Process on Textile Fabrics and Their Effluent Treatment. J Biomed. Nanotechnol. 2007;3:203–208.
CrossRef - Klaus T. J.,Granqvist C. S. Biologically produced silver-carbon composite materials for optically functional thin film coatings. Adv Mater. 2000;12:407–409.
CrossRef - Sharma V. K., Yngard R. A., Lin Y. Silver nano particles: Green synthesis and their antimicrobial activities. Adv Colloid Interfac. 2009;145:83–96.
CrossRef - Vigneshwaran., Ashtaputre N. M., Varadarajan P. V., Nachane R. P., Paralikar K. M., Balasubramanya R. H. Biological synthesis of silver nano particles using the fungus Aspergillus flavus. Mater Lett. 2007;61:1413–1418.
CrossRef - Pandey S., Oza G.,Mewada A and Sharon M. Green Synthesis of Highly Stable Gold Nanoparticles using Momordica charantia as Nano fabricator. Archives of Applied Science Research. 2012;4(2):1135-1141.
- Klaus T., Joerger R., Olsson E., Granqvist C. G. Silver-based crystalline nano particles micro bially fabricated. Proc Natl Acad Sci USA. 1999;96:13611–13614.
CrossRef - Konishi Y., Nomura T., Tsukiyama T., Saitoh N. Microbial preparation of gold nano particles by anaerobic bacterium. Trans Mater Res Soc Jpn. 2004;29:2341–2343.
- Mohanpuria P., Rana K. N.,Yadav K. S. Biosynthesis of nano particles technological concepts and future applications. J Nanopart Res. 2008;10:507–517.
CrossRef - Mandal D., Bolander M. E., Mukhopadhyay D., Sarkar G., Mukherjee P. The use of microorganisms for the formation of metal nano particles and their application. Appl Microbiol Biotechnol. 2006;69:485–492.
CrossRef - Qiaoxin Z., Hao L., Xiaohui W., Xiaoliang S., Xinglong D. Fabrication and Characterization of Nano Silver Powder Prepared by Spray Pyrolysis. J Wuhan Univ Technol. 2009;24:871-874.
CrossRef - Rad S. M.,Rad S. J., Heshmati A.G., Miri A., Sen J. D. Biological Synthesis of Gold and Silver Nanoparticles by Nitraria schoberi Fruits. American Journal of Advanced Drug Delivery. 2013;2:174-179.
- Sony N., Prakash S. Synthesis of gold nano particles by the fungus Aspergillus niger and its efficacy against mosquito larvae. Reports in Parasitology. 2012;2:1-7.

This work is licensed under a Creative Commons Attribution 4.0 International License.





