How to Cite | Publication History | PlumX Article Matrix
Cells Cultivation System and Preparation of the Purple Membranes for Bionanotechnology
Daria O. Solovyeva1, Vladimir A. Oleinikov1 and Sergei Yu. Zaitsev2
1Laboratory of Molecular Biophysics, Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Moscow, RF.
2Biochemistry Department, Moscow SAVMB, Moscow, Russian Federation.
Corresponding Author E-mail: szaitsev@mail.ru
DOI : http://dx.doi.org/10.13005/bbra/2494
ABSTRACT: The purple membranes (PM) of the Halobacterium salinarum cells and bacteriorhodopsin (major retinal-containing trans-membrane protein of these bacteria are the most promising photosensitive biopolymers in terms of bionanotechnology, developing photosensitive devices, etc. The article is devoted to the improvement of the cultivation system for Halobacterium salinarum cells and the PM production; the study of the properties and functional characterization of bacteriorhodopsin - the biological components of the bionanomaterials. The optimal conditions for the production of the biomass are the following: the volume of incubation media must be 10% of the working volume (the volume of the cuvette); the cell “age” must be close to the end of the exponential phase of growth; the lighting must be maximum (4 light bulbs LD-20); the temperature must be 37°С; the time must be 8 days; the aeration must be 0.45 (L air)/(L of environment)/min. The yield of the product is about 5 g of biomass containing 45 mg of bacteriorhodopsin. The purple membranes and their major protein - bacteriorhodopsin were obtained with the required parameters.
KEYWORDS: Cell bionanotechnology; biochemistry of membrane proteins; bacteriorhodopsin nanobiomaterial components; purple membranes;
Download this article as:| Copy the following to cite this article: Solovyeva D. O, Oleinikov V. A, Zaitsev S. Y. Cells Cultivation System and Preparation of the Purple Membranes for Bionanotechnology. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Solovyeva D. O, Oleinikov V. A, Zaitsev S. Y. Cells Cultivation System and Preparation of the Purple Membranes for Bionanotechnology. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=25624 |
Introduction
Modern research in the field of bioelectronics and nanotechnology is often based on the unique properties of biologically active compounds.1-3 The most studied and practically used biologically active compounds are the purple membranes (PM) of the Halobacterium salinarum bacteria and bacteriorhodopsin (retinal-containing transmembrane protein of these bacteria).4-6 Bacteriorhodopsin is one of the most promising photosensitive proteins in terms of developing photosensitive devices (as promising energy sources, optoelectronic elements, storage systems).7-9 That is why the most experiments and developments in bionanotechnology were performed using purple membranes and bacteriorhodopsin as the best examples.10-12
At present, the search for the most effective PM preparations is actively developing. The methods of cultivation of Halobacterium salinarum and the PM production have been described in the literature,13-15 however, it was necessary to “adapt” them to the modern experimental conditions within the present work. The tasks of the recent works are the development and experimental implementation of the technique, which allows to obtain PM preparations in high yield (up to 50 mg/L culture).
It is known that the great amount of halophilic bacteria representatives are able to grow on culture medium, where protein hydrolysates or amino acids are used as the sole carbon source.16,17 At the same time, variation of the salt composition of a culture medium leads to a changes in the level of biomass accumulation, as well as in the shape of Halobacterium cells. Depending on the particular task, a culture medium may have different composition and content of components, but two obligatory components are always the same – sodium chloride in high concentration and some source of amino acids. In general, all kinds of microbial proteins (or biomass) hydrolyzates can be considered as the common sources of amino acids in the cultivation of these halobacteria. An ideal source of the hydrolyzate can be a product of complete acid hydrolysis of milk caseins. The use of cheaper hydrolyzates, including those with an uncontrolled composition, is also possible in the cases where the use of such medium is justified economically.
It is known18 that intense illumination with insufficient aeration of culture leads to an increase in the content of BR in cells, whereas cultivation in darkness with strong aeration leads to inhibition of this protein formation. For this reason, a selection of the conditions (illumination and aeration) is important within this work with the aim of increasing the yield of BR per unit volume of culture liquid (QL), as the cultivation conditions in the fermenter differ significantly from the cultivation in the flasks and represent an independent object of study.
The purpose of this work is to improve the system for cultivating Halobacterium salinarum cells and to obtain purple membranes, to study the properties and functional characterization of the biological components of nanobiomaterials: photosensitive membrane proteins and oriented membranes based on them.
Materials and Methods
The system for Halobacterium salinarum cells cultivation and PM production has been improved at first. In particular, the device (schematically shown in Fig. 1) has been used. The cuvette (1) had about 6 liters volume (Fig. 1) and was made of “plexiglass acrylic sheets” and had height of 40±1 cm, length of 45±1 cm, a thickness of the liquid layer of 3.3±0.1 cm. There were taps for sampling and liquid drain. This system was located in a temperature-controlled box equipped with an aerating device to maintain the temperature, simultaneously, providing stirring. The system had the following parts: air drawn by the pump (20 passes to maintain sterility through the air filter (3), flow-meter (4) and enters the cell (1), where bubbles through the holes of the intake tube) and is lit by four fluorescent lamps LD-20 (5).
Before filling the cuvette with the nutrient medium, it was washed and sterilized by 96% ethanol (sterilization in an autoclave was not possible due to the material from which cuvette was made). To ensure that the aseptic conditions were stable, all the lines connecting the cuvette with other devices were sterilized in the same way. After filling it with 4 liters of sterile growth medium (total volume does not exceed 70% of the total volume of the cuvette), 400 ml (l0% V/V) inoculum was introduced. Before the introduction of the inoculum, the temperature and pH of the medium were adjusted to optimal values for Halobacterium salinarum growth.
Results and Discussion
As a result of cultivation process optimization, a methodology has been developed. According to the developed technique, the cultivation of Halobacterium salinarum was carried out in 4 L of complex medium, containing per 1 liter: 250 g NaCL, 20 g MgS04 • 7 H20, 3 g sodium citrate, 2 g KCL, 2 g yeast extract, 5 g of peptone, 1 ml of glycerol.
After all the salts were dissolved, the yeast extract and peptone glycerin were added to the solution. By titration with KOH solution (10 g/100 ml) pH was set to 7.2-7.4 .The medium was sterilized by autoclaving at 121°C.
For the preparation of the seed material (inoculum) a number of Halobacterium salinarum cells were introduced in vials with a volume of 30 ml containing 5 ml of medium and cultured 5 days at 37°C in a rotational rocking. The amount of 5 ml of seed culture was introduced in Erlenmeyer flasks (750 ml) containing 200 ml of medium and cultivation was continued at rotational rocking (190 min-1) at 37°C. Cultivation of Halobacterium salinarum spent 5 days (until the completion of the exponential phase).
The cultivation and production of material was held directly in the fermenter: 4 liters of medium at 37°C were used with the air flow control to the cultivator; the air flow was set at 1.8 L per min.; the process was carried out under light (four fluorescent lamps LD-20).
The process was carried out in periodic mode in a transparent flat cuvette with a volume of 6 L, illuminated by four fluorescent lamps. Aeration and mixing of culture is ensured by the flow of the air into the lower part of the cuvette (Fig. 1). Cuvette placed in a thermostatic room to maintain the temperature.
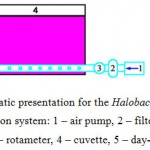 |
Figure 1: Schematic presentation for the Halobacterium salinarum cultivation system: 1 – air pump, 2 – filter for air sterilization, 3 – rotameter, 4 – cuvette, 5 – day-light lamps LD-20. |
The time of periodic fermentation was about two weeks, because of such long time a one of the main requirements (which applied to the fermenter) was the preservation of sterility at intensive aeration of the nutrient medium during the cultivation. So, sterile filters were installed on the air inlet to prevent the ingress of any foreign microorganisms.
The particular increase in the biomass of halobacteria was characterized by the change of optical density at wavelengths of 650 nm absorption (a quantitative characterization of the total content of bacterial mass) and 570 nm (the quantitative content of BR). For this reason, some samples were taken periodically from the cuvette. The sampling volume of about 12 ml was carried out 1 time a day. After determination of the optical density, samples with a volume of 1 ml were centrifuged to precipitate the cells (sediment used to determine the content of BR).
Estimation of the BR content in the halobacterial biomass was carried out according to the following procedure. Some amount of bidistilled water (3 ml containing 1 mg of DNAse per 100 ml) was added to the cells obtained by precipitation from a 10 ml sample. After 3 hours (by completion of DNA hydrolysis) the large cell fragments were precipitated by centrifugation at 10000 g for 10 min. The optical density of the supernatant was determined at 570 nm. The dependences of the optical density vs. the cultivation time were obtained (Fig. 2).
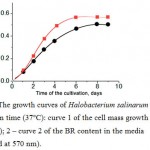 |
Figure 2: The growth curves of Halobacterium salinarum cells vs. cultivation time (37°C): curve 1 of the cell mass growth (measured at 650 nm); 2 – curve 2 of the BR content in the media (measured at 570 nm). |
As a result of various experiments, it was shown that the yield of BR was decreased in the case of the extremely long cultivation time (more than 10 days at 37°C). It should be noted that a two-fold decrease in illumination (two lamps instead of four) during the strain ET1001 cells cultivation caused a proportional decrease in the yield of the BR, while maintaining the same level of biomass accumulation. Therefore, the maximum irradiation intensity (allowed by the dimensions of the cuvette and the design of the lighting devices) was used during cultivation.
The level of biomass accumulation was influenced both by the volume of the seed materials and by the inoculum age. In general, the recommended seed volume of about 5% of the medium inoculated volume was used, but it has been shown that its increase up to 10% caused an increase in the yield of biomass by a factor of 1.3 and a corresponding increase in the yield of BR. The optimal age of the seed material was at the end of the exponential growth phase (5 days).
Thus, optimal conditions for the production of the biomass, when using the strain ET1001 were found: 1) the inoculum volume must be 10% of the working volume (roughly the volume of the cuvette); 2) the age must be the end of the exponential phase of growth; 3) the lighting must be maximum (four light bulbs LD-20); 4) the temperature must be 37°С; 5) the time must be 8 days; 6) the aeration must be 0.45 (L air) / (L of environment) / min. The yield of the product was 5 g of biomass containing 45 mg of bacteriorhodopsin.
To isolate the purple membranes, the cell pellet obtained by centrifugation of 4 L of the liquid was suspended in 100 ml of bidistilled water with included DNAse 1 (1 mg per 100 ml). It was stirred at 4°C for 2 hours. Then, the resulting suspension was dialyzed for 17 hours against 5 liters of bidistilled water at 4°C. To precipitate large cell fragments, the suspension was centrifuged at 4°C at 10000 g for 10 minutes, the precipitate was discarded. The supernatants contained PM were reprecipitated 6 times from bidistilled water by centrifugation at 50000 g at 4°C for 70-120 minutes (depending on the purity of the preparation).
The mass of the BR was determined by the optical method using the optical density values. For this, light adaptation of BR preparations was carried out by illumination for 2 minutes. With the light of a mercury lamp passed through a heat filter and a light filter. Then, the optical density was measured at the chromophore absorption maximum in the light-adapted form of the BR and its mass was calculated by the following formula13:
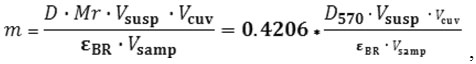
Where D – optical density of the solution at the absorption maximum of the pigment
Mr – BR molecular weight (26700 Da);
V susp – BR suspension volume, ml;
Vcuv – volume of the solution in the spectrophotometric cuvette in ml;
Vsamp – sample volume introduced in the cuvette in ml;
εBR – BR extinction, including the following : 63000 M-1cm-1 at 570 nm or 110000 M-1cm-1 at 280 nm.
According to the developed methods, sufficient quantities of cell cultures and bacterial mass were obtained from which a photosensitive protein bacteriorhodopsin was isolated in an amount of 200 mg.
The purity of the product was monitored spectrophotometrically from the absorption spectrum of the suspension. The ratio of optical densities at 280 nm and 567 nm was independent of protein concentration, but serves as a criterion for its purity. For the obtained product this ratio was 1.45±0.05, i.e. the purity of the product corresponded to the best world parameters. The finished membrane preparations were stored at 4°C in the form of an aqueous suspension with a concentration of about 10 mg protein per ml.
The presence of the chromophore BR retinal was determined from the shape of the absorption spectrum of the produced BR (Fig. 3).
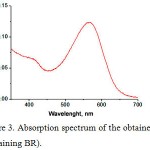 |
Figure 3: Absorption spectrum of the obtained PM (containing BR).
|
There is an absorption peak with a maximum at a wavelength of 570 nm, which corresponds to the basal state of the BR. The recorded absorption spectra completely correspond to the absorption spectra of wild-type BR.
Characterization of the functional properties of the spent BR (Fig. 4) was carried out using a flash photolysis device with Nd-YAG laser excitation (532nm, 7 ns, 15 mJ), a spectral range of 300-900 nm, and a temporal resolution of 0.5 microseconds.11
 |
Figure 4: Kinetic curves of the PM absorption by photoinduced changes in suspension with accumulated BR (at pH 8.5): the measurements were carried out at the wavelengths 410 nm, 500 nm, 550 nm and 630 nm.
|
The photoinduced changes in absorption at 4 wavelengths, characteristic for absorption of the main intermediates of the BR photocycle, were measured (Fig. 4). The measurements were carried out at the following wavelengths: 410 nm, 500 nm, 550 nm and 630 nm. The effective increase in absorption at 410 nm during the first 50 μs after the laser pulse indicates the formation of the intermediate M, which decays during the next 100 ms. This behavior completely corresponds to the characteristics of wild-type BR. Thus, measurements have shown that the obtained BR is fully functional.
Conclusion
As a result of the work, the experimental technique for obtaining a photosensitive membrane protein of bacteriorhodopsin has been improved. The biochemical and cellular equipment was checked and adjusted. Cell cultures and bacterial mass are grown. The membrane protein bacteriorhodopsin was obtained and characterized with the required quality and quantity.
Acknowledgements
The work was carried out with the financial support of the Russian Foundation for Basic Research (grant N.15-29-01193 ofi_m). The authors are grateful to the staff of the Moscow State University (MSU named after M.V. Lomonosov) and National Research Nuclear University (MEPhI) for the equipment, assistance in the measurements, as well as for a fruitful discussion of the material.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- Ch N., Ch M. (ed): Nanobiotechnology. Wiley-VCH-Verlag, Weinheim. 2004;146-167.
- Yu S. Z. Supramolecular Nanosized Systems at the Phase Interface Concepts and Prospects for Bionanotechnologies. Moscow: LENAND.[in Russian]. 2010.
- Zaitsev S. Y., Solovyeva D. O. Supramolecular nanostructures based on bacterial reaction center proteins and quantum dots. Advances in Colloid and Interface Science. 2015;218:34–14.
CrossRef - Zaitsev S. Y., Solovyeva D. O., Nabiev I. Thin films and assemblies of photosensitive membrane proteins and colloidal nanocrystals for engineering of hybrid materials with advanced properties. Advances in Colloid and Interface Science. 2012;183-184:14-16.
CrossRef - Oren A. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 1999;63(2):334–15.
- Birge R. R. Nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biochim. Biophys. Acta. 1990;1016(3):293–35.
CrossRef - Zaitsev S. Y., Kozhevnikov N. M., Barmenkov Y. O., Lipovskaya M. Y. Kinetics of Dynamic Hologram Recording in Polymer Films with Immobilized Bacteriorhodopsin. Photochemistry & Photobiology. 1992;55(6):851-5.
CrossRef - Oesterhelt D., Brauchle C., Hampp N. Bacteriorhodopsin a biological material for information processing. Quart. Rev. Biophys. 1991;24(4):425-54.
CrossRef - Hampp N. Bacteriorhodopsin as a photochromic retinal protein for optical memories. Chem. Rev. 2000;100:1755-22.
CrossRef - Zaitsev S. Y. Membrane nanostructures based on biologically active compounds for biotechnology. Russian Nanotechnology. 2009;4(7–8):6–13. [in Russian].
- Zaitsev S. Y., Lukashev E. P., Solovyeva D. O., Chistyakov A. A., Oleinikov V. A. Controlled influence of quantum dots on purple membranes at interfaces. Colloids and Surfaces B: Biointerfaces. 2014;117:248-4.
CrossRef - Zaitsev S. Y., Solovyeva D. O., Nabiev I. Nanobiohybrid structures based on the organized films of photosensitive membrane proteins. Russian Chemical Reviews. 2014;83(1):38-44.
- Mironova E. V. Biosynthetic application of bacteriorhodopsin analogs. Ph.-D. Diss. Moscow: MITChT. 2002;118.
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667-12.
CrossRef - Kalenov S. V., Baurina M. M., Skladnev D. A., Kuznetsov A. Y. High-effective cultivation of Halobacterium salinarum providing with bacteriorhodopsin production under controlled stress. J. Biotechnol. 2016;233:211-8.
CrossRef - Lanyi J. K. Light energy conversion in Halobacterium halobium. Microbiol. Rev. 1978;42:682-25.
- MacDonald R. E., Green R. V., Lanyi J. K. Light-activated amino acid transport systems in Halobacterium holobium envelope vesicles: role of chemical and electrical gradient. Biochemistry. 1977;16:3227-9.
CrossRef - Sumpper M., Reitmeier H., Oesterhelt D. Biosynthesis of the purple membrane of halobacteria. Angew. Chem. Int. Ed. Engl. 1976;15:187-194.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





