How to Cite | Publication History | PlumX Article Matrix
Vedamanickam R1, Vinoth Kumar2 and Nagendran Dinakaran3
1Department of Medicine, Sree Balaji Medical College and Hospital, Chrompet, Chennai – 600044.
2MGE, Sree Balaji Medical College and Hospital, Chrompet, Chennai – 600044.
3Senior Consultant Gastro Enterologist.
Corresponding Author E-mail: mmdentalcare77@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2518
ABSTRACT: Gastroesophageal reflux disease (GERD) represents a wide spectrum of clinical symptoms. Apart from reflux damage of oescophageal mucosa, it reflects sense of ill feeling and quality of life both physically and psychologically. The key factor in the pathogenesis is exposure of the gastric acid into the oesophageal mucosa in GERD. Various therapeutic modalities are available for the treatment of GERD among which proton pump inhibitors (PPI) hold the key. As a class these drugs are extremely safe and efficient in short term therapy. But reports on its long term efficacy are very few in Indian literature. This study stresses the long term efficacy of PPIs in GERD.
KEYWORDS: Gastroesophageal; pathogenesis; modalities
Download this article as:| Copy the following to cite this article: Vedamanickam R, Kumar V, Dinakaran N. Comparative Clinical Trial on the Efficacy and Tolerability of Esomeprazole Versus Rabeprazole in Gastro Esophageal Reflux Disease – A Long Term Study. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Vedamanickam R, Kumar V, Dinakaran N. Comparative Clinical Trial on the Efficacy and Tolerability of Esomeprazole Versus Rabeprazole in Gastro Esophageal Reflux Disease – A Long Term Study. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=25939 |
Methodology
Study Design
Open comparative clinical study of Esomeprazote versus Rabeprazole in patients with GERD and its long term follow up.
Materials and methods
This study was carried out after obtaining the informed consent from the patients for the drug trial.
Study population
Total no. of patients – 140
Sex – Both sexes
Age group — 18 to 58 years.
Drug Selection
Either Esomeprazole 40 mg orally or Rabeprazole 20 mg;given once daily before breakfast for the initial 8 weeks.
Patient Evaluation
Baseline symptoms like heart burn, regurgitation and abdominal bloat were assessed on a 4 point clinical scale from 0 (no symptoms) to 3 (severe symptoms) and endoscopic grading (Grade 0 — normal mucosa, Grade 1— erythema or hyperemia of the oesophageal mucosa, Grade 2 — superficial ulceration or erosion involving less than 10% of the surface of the distal 5 cm of oesophageal mucosa, Grade 3 — superficial ulceration or erosion involving less than 10 to 50 % of the surface of the distal 5 cm of oesophageal mucosa and Grade 4 — deep ulceration anywhere in the oesophagus or confluent erosions involving more than 50 % of the mucosa’ surface of the distal 5 cm of oesophagus).
Exclusion Criteria
Patients who were pregnant or breast feeding.
Patients with oesophageal stricture or gastric outlet obstruction.
Post operative stomach.
Patients with malignancy.
Severe systemic diseases.
Patients on long term NS AIDs.
Known hypersensitivity to these drugs.
Treatment and follow up
Phase I
Symptoms and endoscopic findings were graded on a scale from Grade 1 to 4 as mentioned before both at baseline before starting drug therapy and after 8 weeks Any complications or side effects of the drugs were also recorded and the patients were instructed to report within 48 hours in such situations.
Phase II
After discontinuation of therapy, these patients were followed up for the next 24 months for symptom analysis. These patients were requested to report at the end of 6th month. 12th month, 18th month and 24th month and their symptoms were recorded.
Phase III
During the follow up, if the symptoms recur, the grading of symptoms were recorded and the patients were restarted on the same drugs they were earlier on at the start of the trial. The drugs were given for the next 8 weeks and the efficacy recorded. Any minor side effects, which do not require discontinuation of therapy, were also carefully recorded. UGI endoscopy was repeated in whom satisfactory relief was not obtained at the end of study period.
Criteria for Evaluation
The severity is assessed both clinically and endoscopically.
Clinical Assessment
Grade 0 — Complete symptom resolution.
Grade 1 — Improvement of symptoms, but not total resolution.
Grade 2 — No change of symptoms.
Grade 3 — Worsening of symptoms.
Endoscopic Assessment
Grade 0 — Complete healing. Grade I — No visible macroscopic erosion, but erythema present. Grade II — Superficial ulceration and erosion involving < 10% of mucosal surface. Grade III — Ulceration or erosion involving 50% of mucosal surface at the distal 5 cm. Grade IV — Deep ulceration in the distal 5 cm of oesophagus.
Withdrawal of Patients from the Study
Request of the patient.
Discretion of the principal investigator on clinical grounds.
Patient lost for follow up.
Adverse side effects.
Results
Demographic Data
Total no. of patients — 140.
Age group — 18 — 58 years.
Sex — both sexes.
Rabeprazole group — 70 patients.
Esomeprazole group — 70 patients.
Phase I Trial
Symptom Analysis
There was significant decrease in the clinical severity of symptoms in both the groups, 70% in Esomeprazole group and 84% in Rabeprazole group at the end of 2″ week. At the end of 8th week 90% relief was noted with Esomeprazole and 92% relief was noted in Rabeprazole group.
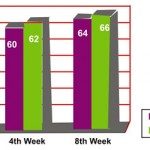 |
Figure 1
|
Healing Rate of Oesophagitis by Endoscopy
Table 1
| Grade | Esomeprazole | Rabeprazole | ||
| Before Treatment | No. responded (%) | Before Treatment | No. responded (%) | |
| I | 35 | 32 (91%) | 31 | 28 (90%) |
| II | 30 | 25 (83%) | 32 | 27 (84%) |
| III | 5 | 3 (60%) | 7 | 4 (57%) |
| IV | – | – | – | – |
| Total | 70 | 60(84%) | 70 | 59 (82%) |
UGI endoscopy revealed 84% healing rate by Esomeprazole group and 82% healing rate in Rabeprazole group.
Tolerability
In Esomeprazole group 7% and 5.6% in Rabeprazole group had bearable side effects like mild giddiness and head ach. No body had severe side effects as to warrant stoppage of treatment.
Phase II
During the post treatment follow up in the next 24 months following is the observation made in symptom analysis.
Clinical Improvement of Symptoms
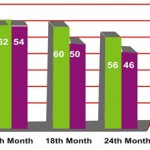 |
Figure 2
|
At the end of 24 months 78% in Esomeprazole group and 64% in Rabeprazole group . were symptom free.
Phase III
In patients who had recurrence of symptoms, the same drugs were reintroduced. In the Esomeprazole group 11 patients and in the Rabeprazole group 24 patients had recurrence of symptoms. The following observations were made in them.
Table 2
| Period | Esomeprzole-Total 14 | Rabeprazole – Total 24 | ||
| No. responded | % | No. responded | % | |
| 2nd week | 7 | 50% | 14 | 58% |
| 4th week | 8 | 57% | 16 | 66% |
| 8th week | 13 | 92% | 20 | 83% |
No relief of symptoms was noted in 8% of patients in the Esomeprazole group and 17% of patients in the Rabeprazole group. Resistance to therapy in both the groups was associated with large hiatus hernia and in elderly age group.
Discussion
Esomeprazole has shown, in recent trials to be more effective in control of intragastric pH, higher and more predictable ulcer healing rate and maintenance of virtually all the patients in remission, when compared to Rabeprazole. The metabolism1 and pharinacokinetic profile2 of the PPI polymorphism plays an additional role in the therapeutic effectiveness of PPI during the treatment of acid related disorders. PPI act by binding covalently to the aminoacid cystein 813 present in the primary binding site in the proton pump in the luminal surface of the gastric parietal cells. This results in the irreversible inhibition of acid secretion. The acid secretion resume only after recruititjg newly synthesized proton pumps from the Golgi apparatus. Esomeprazole is more potent than Rabeprazole in long term treatment of GERD.5,6
Conclusion
Oral Esomeprazole is as effective as Rabeprazole in GERD patients during short term therapy.7 Symptomatic relief was sustained in 78% of patients in Esomeprazole group when compared to 64% in Rabeprazole group. Resistance to treatment occurs in 8% of patients in Esomeprazole group, when compared to 18% in Rabeprazole group. when reintroduced in patients with recurrence of symptoms. Esomeprazole has the longest area under the curve and gives acid suppression for the longest period of time8 So it is very effective in the long term treatment of GERD patients. Side effects are insignificant with both drugs.
References
- Ishizaki T., Horai Y. Aliment. Phamacol. The. 1999;13(3):27-36.
- Scott M. Gastroesophageal reflux disease : Diagnosis and management. American Family Physician. 1999;59(5):1161 —9.
- Sach G. Improving on PPI based therapy of GERD. Eur. J. Gastroenterol. Hepatol. 2001;13:535—40.
- Johnmsson L et al. Timing of reflux symptoms and esophageal acid exposure. Gullet. 1992;2:58—62.
- Smith W.,et al. Esomeprazole 40 mg provides more effective acid control than Rabeprazole 20 mg. Gut. 2000;47(3).
- Smith W.,et al. Esomeprazole 40 mg provides more effective acid control than Rabeprazole 20 mg in patients with symptoms of GERD. Am. J. Gastroenterol. 2001;96(5):345.
- Richter. J. E., Kahsilar P. J., et al. Efficacy and safety of Esomeprazole. Am. J. Gastroenterol. 2001;96:656—65.
- Hattlebakk J. G et al. Medical therapy : Management of refractory patient. Gastroenterol. Clin. North. Amer. 1999;28:847—60.
- Dent J et al. An evidence based appraisal of reflux disease management — The Geneva work shop report. Gut. 1999;44 (2):51—516.

This work is licensed under a Creative Commons Attribution 4.0 International License.





