How to Cite | Publication History | PlumX Article Matrix
In vitro Regeneration of Alstroemeria cv. ‘Balance’ Based on Direct Organogenesis
Maryam Beigi Harchegani1, Hossein Nazarian2, Mahmoud Otroshy3, Mohammad Ali Ebrahimi4 and Ali Motamedi5
1,2Department of Agricultural Biotechnology, Payame Noor University, Karaj, I. R. Iran.
3Department of Tissue Culture, Agricultural Biotechnology Research Institute of Iran, Najaf Abad, Isfahan, Iran.
4Department of Agricultural Biotechnology, Payame Noor University, Tehran, I. R. Iran.
5Faculty of Agriculture, Shahid Beheshti University, Tehran, Iran.
Corresponding Author Email: alimotamedi1987@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2479
ABSTRACT: The present study aimed at improving the efficiency of the in vitro regeneration of Alstroemeria cv. ‘Balance’ protocol through direct organogenesis technique using three different origins of explants (nodal stem, rhizome apical bud, rhizome segments) as two separate factorial experiments with completely randomized design with six replications which were implemented in three stages. Firstly, Direct regeneration, including two factorial experiments to induce regeneration in organogenesis media by utilizing the four NAA concentrations (0, 0.5, 1, 2 mg/l) combined with five BAP concentrations (0, 0.5, 1, 1.5, 2 mg/l) in the first experiment, and TDZ concentrations (0, 0.5, 10 mg/l) in combination with three IBA concentrations (0, 1, 2 mg/l) in the second experiment. Secondly, shoot regeneration and elongation of stems in regenerated buds in the MS basal medium and finally, rooting of the regenerated shoots in the root induction mediums by NAA and IBA. Results of regeneration experiment revealed that, in the culture medium containing 10 mg/l TDZ in combination with 2 mg/l IBA and rhizome apical bud explants, the maximum rates of regeneration percentage, the highest number and length of the shoots were produced. Resulted shoots produced the greatest number of roots and rhizomes in the culture medium comprising 1 mg/l NAA in combination with 2 mg/l IBA as auxins. From the two tested explants, rhizome apical bud, was known as the best explants for shoot regeneration obtained plantlets successfully adapted to environmental conditions and were transferred to the greenhouse. Results of current study indicated that Alstroemeria can be produced through direct organogenesis.
KEYWORDS: Alstroemeria; direct organogenesis; tissue culture; plant growth regulator; explants
Download this article as:| Copy the following to cite this article: Harchegani M. B, Nazarian H, Otroshy M, Ebrahimi M. A, Motamedi A. In vitro Regeneration of Alstroemeria cv. ‘Balance’ Based on Direct Organogenesis. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Harchegani M. B, Nazarian H, Otroshy M, Ebrahimi M. A, Motamedi A. In vitro Regeneration of Alstroemeria cv. ‘Balance’ Based on Direct Organogenesis. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=25843 |
Introduction
Alstroemeria is a cross-pollinating plant from Alstroemeriaceae Family (Khaleghi et al., 2008). And is a rhizomatous monocotyledon (Chiari and Bridgen, 2000), Which, due to the beautiful flowers and long shelf life of its cut flowers, is considered as one of the most important ornamental plants (Hamidoghli et al., 2007). This plant is propagated vegetatively through rhizome splitting (Healy and Wilkins, 1981). But its propagation rate is low and it faces seasonal time limits. To remove this limitation, in vitro culture system based on bud culture and rhizome meristems have been developed. In order to overcome these limitations and to produce virus-free plants, in vitro culture methods have been taken under advisement (Khaleghi et al., 2008). Apical shoot bud (pedraza et al., 2006), lateral shoot bud (Lin et al., 1997), and rhizome (Lin et al., 2000) have been utilized for in vitro propagation of Alstroemeria. Possibility of organogenesis from leaf and stem has not been considered in monocotyledons. The development of leaf and stem culture system in Alstroemeria is valuable, although successful organogenesis from leaf and stem is strongly genotype-dependent at the present. These results could expand the possibilities of using of culture systems for Alstroemeria (Hoshino, 2008).Cells, tissues and organs selected from multiple plant species could successfully be cultured and complete plants may be produced by them. Some researchers have reported that using rhizome apical bud as explant is more suitable than peduncle and vegetative stems (Lin et al., 1987). Researchers in the studies of direct regeneration of the shoots through leaf explants obtained from seedlings being grown in vitro conditions, reported that the best induction was achieved in MS basal medium containing 2.2 mg/l BAP, 10 mg/l TDZ and 0.5 mg/l IBA (Lin et al., 1998). Thus, this study aimed at achieving the best explants and plant growth regulators in order to induce regeneration in Alstroemeria cv. ‘Balance’. In the present study, the effects of various concentrations of BAP, TDZ, NAA, and IBA, were for the first time investigated in combination with origins of explants for regeneration induction and following, shooting and root formation obtained from aerial organs in Alstroemeria.
Materials and Methods
Culture Medium
In this study, MS basal medium was utilized as the culture medium. 30 g sucrose and 7 g/l of agar were added to the culture mediums. Before autoclaving and agar addition, the media were pH 5.8 adjusted. In the first experiment of direct regeneration, BAP plant growth regulator with concentrations of 0, 0.5, 1, 1.5, 2 mg/l in combination with 0, 0.5, 1, 2 mg/l NAA and in the second experiment of direct regeneration (Shoot induction and elongation of stem in regenerated buds) TDZ plant growth regulator was utilized with concentrations of 0, 0.5, 10 mg/l in combination with concentrations of 0, 1, 2 mg/l IBA.
Plant Materials
Explants from nodal stem, rhizome apical bud and rhizome segments of Alstroemeria cv. ‘Balance’ were prepared.
Explants Preparation
To disinfectthe rhizome segments and nodal stem, surface rinsing with running tap water was implemented for 20 min in order to remove surface pollutions. After washing the explants, disinfection of rhizome segments and nodal stem was done using with 70% Ethylic alcoholrespectively, for 30 seconds and 1 min. Then, concentration of 3.5%sodium hypochlorite was used for 20 min for nodal stem and concentration of 20% sodium hypochlorite was utilized for rhizome disinfection for 35. After each step of disinfection, explants were washed in sterile, distilled water and following the last stage of sterilization, they were rinsed three times in sterile, distilled water.
Disinfection stages were done in the laminar flow cabinet and each culture jar contained 4 explants.To prepare the nodal stem explants, after removal of young stems from plants, the upper and lower parts of stem nodes were cut into pieces by scalpel with a length of 1 cm and the explants were placed vertically in culture mediums. The culture jars were incubated in growth chamber whose environmental conditions were adjusted to 22±2°C, with photoperiod of 16 h light to 8 h dark per day. In the all of direct organogenesis experiments, light intensity was set at the level of 3000 lux. After 30 days, the percentage of regeneration was measured and buds were transferred to fresh media for shoot induction.
Shoot Induction and Elongation of Stems
Obtained buds from direct organogenesis (previous stage) were transferred to fresh media with the same PGRs concentrations to produce stems. After subculture of explants in the culture jars containing 30 mg culture media, the number and length of the shoots were measured30 days after shoot induction and elongation of stems.
Assessed of Root Induction Treatments
In the experiment of root induction, IBA with concentrations of 0, 1, 2 mg/l were evaluated in combination with the concentrations of 0, 0.5, 1 mg/l NAA. Regenerated organs (obtained shoots without roots) were transferred to root induction media and after 21 days, the rooting percentage, length of the highest root, the average of root number and the number of the rhizomes were measured.
Hardening and Transferring to the Greenhouse
After 9 weeks, plantlets with developed roots and rhizomes were transferred in to plastic pots contained Peat Moss and Perlite (2:1). After that, pots were transferred to the baskets covered with plastic and were sprayed with water for two times a day and allowed to grow at 24oC with 16 h photoperiod. In the second week, small hole were made on the plastic cover and from the third week, the covers were removed gradually. Finally, plantlets were transferred to the greenhouse after adaptation processes.
Statistical Analysis of Direct Regeneration and Rooting
These experiments were conducted in a separate factorial design with 3 factors with six replications. The obtained data were standardized utilizing “Subtract mean and divide by standard deviation” in Minitab software version16.2. Analysis of variance (ANOVA) was done using Duncan’s mean comparison at the probability levels of 0.05, and 0.01 by the SAS software version 9.2.
Results
Due to the lack of proper response in the first three experiments of regeneration, no significant result was obtained and analysis of variance was not needed. The experiments were followed using rhizome apical bud and rhizome segments, owing to the absence of nodal stem explants reaction to regeneration by three months and because of chlorosis, senescence and degeneration of explants, which could be attributed to secretion endogenous and exogenous ethylene.
Effects of TDZ, IBA Levels and Explants on Percentage of Regeneration
According to the analysis of variance effect of PGRs and origin of explants, individually, and also interaction of PGRs had significant effects on percentage of regeneration (P≤0.01 (Table 1). Among the various levels of TDZ, the concentration of 10 mg/l had the highest effect on percentage of regeneration (34.56%) and among the different levels of IBA, the concentration of 2 mg/l had the maximum impact on regeneration (38.89%) (Table 2). Also rhizome apical bud showed most percentage of regeneration (26.84%). Interaction of PGRs without considering the explants (Table 3) revealed that, MS basal medium with 10 mg/l TDZ in combination with 2 mg/l IBA caused the greatest percentage of regeneration (66.67%). The interaction of explants and each PGRs, separately and also interaction of PGRs and explants were not significant (Table 1).
Table 1: Variance analysis of TDZ, IBA and Explants’ effects on the measured parameters in regeneration
| Source | df | regeneration | Mean square the number | shoot length (mm) |
| percentage% | of shoots | |||
| TDZ | 2 | 166.97** | 125.59** | 210.86** |
| IBA | 2 | 188.57** | 167.13** | 206.13** |
| Explant | 1 | 74.87** | 49.01** | 0.05ns |
| TDZ*IBA | 4 | 41.37** | 64.99** | 48.93** |
| TDZ*explant | 2 | 14.62ns | 14.38ns | 06.05ns |
| IBA*explant | 2 | 10.47ns | 08.68ns | 12.54ns |
| TDZ*IBA*explant | 4 | 0.15ns | 0.58ns | 09.10ns |
| Error | 83 | 8.27 | 7.99 | 16.05 |
| CV% | 21.76 | 22.77 | 30.6 |
Table 2: Means comparison of TDZIBA and explants’ effects on the measured parameters in regeneration
| TDZ | regeneration% | number of | length of | IBA | regeneration% | number of | length of | explant | regeneration% | number of | length of |
| (mg/L) | the shoots | the shoots | (mg/L) | the shoots | the shoots | the shoots | the shoots | ||||
| 0 | 4.85c | 0c | 0.24b | 0 | 11.03b | 0.2b | 0.43b | rhizome | 26.84 a | 0.7a | 1.88a |
| apical | |||||||||||
| bud | |||||||||||
| 5 | 26.39 b | 0.67b | 2.26a | 1 | 14.57b | 0.5b | 1.80b | rhizome | 16.35b | 0.5b | 1.91a |
| 10 | 34.56a | 1.23a | 2.27a | 2 | 38.89 a | 1.28a | 3.38a | segments |
Effects of TDZ, IBA and Origin of Explants on the Number and Length of the Shoots
Based on the results of analysis of variance shown in Table 1, PGRs each separately had a significant effect on the average of number and length of the shoots(P≤0.01).Among different levels of TDZ, the concentration of 10 mg/l had the greatest impact on average number (1.28) and length of the shoots (2.27 cm) and from the different levels of IBA, concentration of 2 mg/l had the highest impact on the average number (1.28) and length of the shoots (3.38)(Table 2). According To the interaction between growth regulators regardless of the type of explants, in the MS culture medium with 10 mg/l TDZ combined with 2 mg/l IBA, the highest number (2.57) and length of the shoots (6) were observed(Table 3). There was a significant effect of explants on the average number of the shoots (P≤0.01); but no significant effect on the average length of the shoots(Table 1). Amongst evaluated explants, rhizome apical bud indicated the highest average number of the shoots(0/7) (Table 2). Interaction between explants and each of thePGRs alone, and also interaction of explants combined with PGRs, had not significant differences on the number and length of the shoots (Table 1).
Table 3: Means comparison of TDZ and IBA interaction effects on the measured parameters regeneration
| number of the shoots | length of the shoots | |||||||||
| regeneration% | IBA (mg/L) | |||||||||
| 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | ||
| TDZ (mg/L) | 0 | 0c | 2.07c | 12.5c | 0c | 0.07c | 0.24bc | 0c | 0.41c | 0.42c |
| 5 | 18.74bc | 22.92bc | 37.50b | 0.24bc | 0.74bc | 1b | 0.47c | 2.58bc | 3.73ab | |
| 10 | 15bc | 18.74bc | 66.67a | 0.35bc | 0.67bc | 2.57a | 0.88c | 2.63bc | 6a |
Effects of Different Levels of NAA, IBA And Origin of Explants on Rooting
According to the comparison of the mean, different concentrations of NAA and IBA as auxin, also effect of explants, interaction of PGRs (NAA and IBA) and interaction of PGRs combination with explants, revealed significant differences on percentage of rooting (P≤0.01)(Table 4).As the comparison of the means showed (Table 5), 1 mg/l NAA (38.96%) and concentration of 2 mg/l IBA (27.79%), had the greatest effect on rooting. Rhizome apical bud explants ranked statistically as category ‘a‘ (25.45%). MS basal medium containing 1mg/l NAA in combination with 2 mg/l NAA, without considering the origin of explants was the best treatment with 62.5%(Table 6). As the Diagram2shows, culture medium containing 1 mg/l NAA in combination with 2 mg/l IBA with rhizome apical bud explants, had shown the greatest rooting (91.66%).As shown (Table 4), interaction of each PGR, separately with explants, had significant differences (P≤0.05).Comparison of the means (Diagram 1) revealed that culture medium containing 1 mg/l NAA with rhizome apical bud and without presence of IBA, indicated the most percentage of rooting (25%) and culture medium contained 2 mg/l IBA, rhizome apical bud and without presence of NAA, the highest percentage of rooting was obtained (16.67).
Effects of Different Levels of NAA, IBA and Origin of Explants on the Length of the Root
According to analysis of variance (Table 4), the effects of different concentrations of NAA, IBA auxins, and interaction of NAA and IBA, had significant effects on the average of highest length of the roots (0.01≤P). Concentration of 1 mg/l NAA (3.88 cm) and concentration of 2 mg/l IBA with rooting percentage of 2/58 cm had the greatest impact on the average of highest length of the root (Table 5). The effect of explants was significant (P≤0.05) (Table 4). Rhizome apical bud ranked statistically as category ‘a'(2.27 cm) (Table 5). As shown in Table 6, the culture medium containing 1 mg/l NAA in combination with 2 mg/l IBA, without considering the type of explants, had the greatest effect on the average of the highest length of the root. Interaction of each PGR and explants, also interaction of combination of PGRs and explants were not significant (Table 4).
Table 4: Variance analysis of NAA, BAP and Explants’ effects on the measured parameters in rooting
| Source | df | root formation | Mean square the highest | the average number of the root | the average number of |
| percentage% | root length | the root | |||
| NAA | 2 | 195.08** | 278.45** | 162.18** | 228.87** |
| IBA | 2 | 50.23** | 74.14** | 44.32** | 90.70** |
| Explat | 1 | 74.72** | 38.90** | 22.80* | 50.46** |
| NAA*BAP | 4 | 62.10** | 46.92** | 33.15** | 48.96** |
| NAA*explant | 2 | 19.51* | 06.20ns | 09.96ns | 25.58ns |
| BAP*explant | 2 | 18.59* | 13.32ns | 05.55ns | 11.76ns |
| NAA*BAP*explant | 4 | 23.63** | 05.31ns | 04.39ns | 05.23ns |
| Error | 83 | 6.02 | 37.1 | 17.6 | 5.72 |
| CV% | 18.92 | 24.07 | 19.62 | 9.1 |
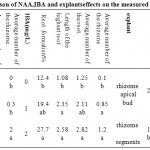 |
Table 5: Means comparison of NAA,IBA and explants effects on the measured parameters in rooting
|
Effects of Different Levels of NAA, IBA and Origin Of Explants on Average Number of the Roots
As the results of analysis of variance NAA and IBA auxins separately, also the interaction of these PGRs had significant effects on the average number of the root (P≤0.01) (Table 4). Among different concentrations of NAA, concentration of 1 mg/l caused the highest average number of the root (4.09). Among different concentration of IBA, greatest average number of the root, was observed in concentration of 2 mg/l IBA (2.82)(Table 5). The culture medium containing 1 mg/l NAA in combination with 2 mg/l IBA ranked statistically as category ‘a’ (5.96) (Table 6).
Tale 6: Means comparison of NAA and IBA interaction effects on the measured parameters in rooting
| IBA (mg/L) | root formation% | the average number of the root | the highest number of the root | the average number of the rhizome | |||||||||
| 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | 0 | 1 | 2 | ||
| NAA (mg/L) | 0 | 0 | 4.17 | 14.57 | 0c | 0.27 | 0.62c | 0d | 0.37 | 1.67 | 0 | 0 | 0 |
| c | bc | bc | cd | cbd | c | c | c | ||||||
| 0.5 | 12.19 | 27.07b | 6.24 | 0.33 | 3.61 | 2 | 1.12 | 2.77 | 1.32 | 0 | 0.46c | 0.67c | |
| bc | bc | c | ab | bc | cbd | cb | cbd | c | |||||
| 1 | 22.82b | 27.07b | 62.5 | 3.27ab | 2.07bc | 5.08a | 2.76cb | 3.17b | 5.96 | 0/64c | 2.12b | 3.23a | |
| a | a |
The effect of explants was significant (P≤0.05) (Table 4).Rhizome apical bud ranked statistically as category ‘a'(2.31) (Table 5).Interaction of each PGR with explants and interaction of the combination of PGRs and explants were not significant (Table 4).
Effects of Different Levels of NAA, IBA and Origin of Explants on The Average Number of The Rhizome
Based on the results of analysis of variance, effect of each PGR separately, and interaction of PGRs, had significant differences on the average number of the rhizome (P≤0.01))(Table 4). Among concentrations of NAA, concentration of 1 mg/l had the most effect on the average number of the rhizome. Among the concentrations of IBA, concentration of 2 mg/l had the most effect on the same parameter. Rhizome apical bud had the greatest effect on the average number of the rhizome (Table 5).Concentration of 1 mg/l NAA in combination with 2 mg/l IBA, regardless to the effect of explants, had the greatest impact on average number of the rhizome (3.23)(Table6). Interaction of explants and each PGR, also interaction of the combination of PGRs with explants, were not significant (Table 4).
Hardening and Transferring to the Greenhouse
After growth and rooting stages, in order to harden them, the plantlets with developed roots and rhizomes were transferred into plastic pots. After one month, 76% of plantlets were transferred to greenhouse following adaptation processes.
Discussion
In the first experiment of regeneration, only a case of regeneration (25%) was observed, which is not debatable and revealed that, these PGRs as mentioned concentrations did not have any positive effects on regeneration induction in rhizome apical bud and rhizome segments. Contrary to our results, Hamidoghli et al. (2007) reported that the culture medium containing 0.5 mg/l BAP and 0.2 mg/l NAA was the best PGRs treatment on the propagation of Alstroemeria cv. Jamaica. The treatments containing TDZ, solely or in combination with IBA, had a positive response to regeneration which indicated a special effect of this PGR on differentiation and bud induction. A considerable point in the effect of TDZ on induction of regeneration was the positive impact of this cytokinin-like substance (TDZ) singly or in combination with IBA on regeneration. It can be suggested that, the increasing concentration of TDZ, had a positive impact on regeneration, which rose as the concentration of IBA increased. TDZ effectively in the induction of regeneration on shoots from leaf explants of many Dicotyledonous were utilized (Tuk, et al., 1994; Dubois and Vries, 1995; Huetteman and Preece, 1993). However, such an effect was not reported on monocotyledons by any researchers, except Lin et al. (1998) who reported that in MS basal medium containing 2.2 mg/l BAP, 10 mg/l TDZ and 0.5 mg/l IBA with leaf explants caused a response to regeneration and bud induction.
Since the effect of cytokinin singly was not very effective on increasing the number and length of the shoots, and this effect was more prominent in combination with auxin, the essential role of auxin in these parameters can be pointed. Similar to our results, George et al., (2008) reported theauxin also caused an increase of apical dominant, which is essential in growth process and shoots elongation. However our finding were different from those of Pierik et al (1997), which reported that auxin was not effective in shoot growth. Hamidoghli et al (2007) reported increasing the concentration of BAP, caused a decrease in shoot length due to reduction in apical dominant. PGRs-free culture medium significantly affected the decline in the length of the shoots, and as a result, inhibition of regenerated bud growths. No more studies on the length of shoots obtained from regeneration are available.
Some reports suggested that NAA is an effective PGR in rooting. Christensen et al. (1999) similarly reported that NAA stimulates induction of the roots, which was consistent with our results. In some of species, adventitious roots form in the proliferation of shoots. While in other species, root formation occurs when they are transferred to cytokine in-free medium. However, some species need special circumstances that usually necessitate auxin presence in culture medium. Cytokinins generally inhibit root formation. Thus they do not exist in rooting medium. Therefore, an intermediate step of transfer to hormone-free medium may be useful (Smith). As auxins may stimulate rooting, consequently, it can prevent root growth; thus, exposing the cultures temporarily, may be effective (Smith). The auxins which are often added to rooting medium contain 0.1 to 10 mg/l IAA, 0.05 to 1 mg/l NAA and 0.5 to 3 mg/l IBA. Khaleghi et al (2008) showed that the type of utilized cytokinin did not affect the number of buds and regenerated roots by rhizome explants and rooting was primarily influenced by the type and concentration of utilized auxin, which is in agreement with our report stating that NAA and IBA were effective in rooting.
Considering the maximum length of roots in high concentrations of both auxins as well as significant increase in the combination of two auxins, essential role of auxins on increasing the roots can be suggested.
Regarding the results of average number of root and rhizome, it can be deducted that a decrease in the concentration of IBA led to a decline the number of root and rhizome. Hamidoghli et al., (2007) indicated that the highest number of roots was obtained utilizing rhizome explants derived from in pot plants circumstances in presence of 0.2 mg/l NAA. Similar results were reported by Podowinszka et al (1997), in which they reported that NAA application in culture medium extremely stimulated root formation. Gabriszewska and Hempel (1985) reported that BA increase could stimulate rhizome proliferation. Pierik et al. (1997); Gabriszewska and Hempel (1985), reported that among cytokinins, BAP is stimulated the proliferation of rhizome, but NAA and IBA did not have effects on this parameter. This did not accord with our reports which showed that concentration of 1 mg/l NAA in combination with 2 mg/l IBA boosted the average number of the rhizome. It can be suggested that due to the application of appropriate concentrations of PGRs has been mentioned in our tests. Han et al (1994), also reported that medium supplemented with 1-2 mg/l BA in combination with 0.2 mg/l NAA was showed the highest number of rhizome.
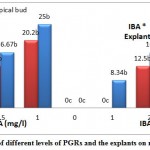 |
Figure 1: Effects of different levels of PGRs and the explants on rooting percentage Click here to View figure |
Means with the similar letters are not significantly different at 5% level of probability using Duncan’s test
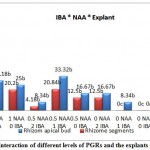 |
Figure 2: Interaction of different levels of PGRs and the explants on rooting Click here to View figure |
Means with the similar letters are not significantly different at 1% level of probability using Duncan’s test
Conclusions
In studies conducted on tissue culture of Alstroemeria, there have been no reports available on comparing different concentrations of TDZ cytokinin. Based on the result of this research, it was concluded that, TDZ had a unique effect on the induction of regeneration, compared with BAP. Another point in this case is the effect of explants in combination with cytokinins on regeneration process. As it was deducted from the results rhizome apical bud was the best explants for regeneration induction. Culture medium containing 10 mg/l TDZ in combination with 2 mg/l IBA and rhizome apical bud explants resulted in the greatest effect on percentage of regeneration (72.17%), and the largest number and length of the shoots. Therefore, it was known as the best culture medium. Derived shoots in the culture medium containing 1mg/l NAA in combination with 2 mg/l IBA, which was introduced as the best rooting culture medium produced the highest number of root and rhizome. Since no measures have been taken in the field of tissue culture of Alstroemeria cv. ‘Balance’ so far, introduction of this genotype in production cycle by modern proliferation procedures (in vitro culture) compared with traditional and common methods could be a great innovation in the mass production of this plant.
Acknowledgements
This research was partially supported by Payame Noor University of Iran. We thank our colleagues from biotechnology laboratory that provided insight and expertise that greatly assisted the research, although they may not agree with all of the interpretations of this paper.
Conflict of Interest
All other authors report no conflicts of interest relevant to this article and the conflicts.
References
- Smith A. R. Html.plant tissue culture techniques and testing services, Translation: Bagheri, e. And c. the freedom. Press Mashhad University Jihad. 2002.
- Tours k. for plant tissue culture techniques gardening (Bvstandary) the translation of politeness, d. Shiraz University Press. 1998.
- Tours k. for plant tissue culture techniques gardening (Bvstandary), the translation of politeness, d. Shiraz University Press. 1998
- Khaleghi.,Sahraroo A., Rasoulnia I. N,, Ataei R. In vitro propagartion of Alstromeria cv. ‘Fuego’. Am-Euras. J Agric Environ Sci. 2008;3:492-497.
- Chiari A., Bridgen M. P. Rhizome splitting: a new micropropagation technique to increase in vitro propagule yield in Alstroemeria. Plant Cell Tiss Org Cult. 2000;62:39-46.
CrossRef - Lin H. S., De Jeu M. J and Jacobsen E. Direct shoot regeneration from excised leaf explants of invitro grown seedlings of Alstroemeria L. Plant Cell Rep. 1997;16:770-774.
CrossRef - Lin H. S., De Jeu M. J & Jacobsen E. Formation of shoots from leaf axils of Alstroemeria the effect of the position on the stem. Plant Cell Tiss. Org. Cult. 1998;52:165–169.
CrossRef - Lin H. S., De Jeu M. J & Jacobsen E. The application of leafy explant micropropagation protocol in enhancing the multiplication efficiency of Alstroemeria. Sci. Hortic. 2000;85:307–318.
CrossRef - Lin W. C., Monette P. L. In vitro propagation of AlstroemeriaAlsaan. Plant Cell Tiss Org Cult. 1987;9:29-35.
CrossRef - Yoichiro H. Advances in Alstroemeria Biotechnology. Hokkaydouniversity. Floriculture, Ornamental and plant Biotechnology. 2008(5):540-547. 978-4-903313-12-2.
- Hamidoghli Y., Bohloli S., Hatamzadeh A. In vitro propagation of Alstroemeria using rhizome explants derived in vitro and in pot plants. Afr J Biotechnol. 2007;6:2147-2149.
CrossRef - Huetteman C. A and Preece J. E. Thidiazuron apotent cytokine in forw culture. Plant Cell Tissue Organ Cult. 1993;33:105-119.
CrossRef - Dubois L. A. M and de Vries D. P. Preliminary report on the direct regeneration of adventitious buds on leaf expiants of in vivo grown glasshouserose cultivars. Gartenbauwissenschaft. 1995;60:249-253.
- Tuk B. A., Swartz H. J and Zimmerman R. H. Adventitious shoot rgeneration from in vitro cultured leaves of Rubus genotypes. Plant Cell Tissue. 1994;56:113-124.
- George E. F., Hall M. A., Klerk G. J. D. Plant Propagation by Tissue Culture. 3rd Edition, Springer. 2008;175-204. DOI 10.1007/978-1-4020-5005-3.
CrossRef - Podwysznska M., Gabryszewska E & Przybyla A. Micropropagation of Alstroemeria x hybrida ‘Juanita’. Acta. Hortic. 1997;447:175–177.
CrossRef - Gabryszewska E & Hempel M. The influence of cytokinins and auxins on Alstroemeria in tissue culture. Acta. Hortic. 1985;167:295–300
CrossRef - Han B. H., Kim Y. J and Choi J. K. Micropropagation ofAlstroemeria through rhizome bud culture. J. Korean Soc. Hortic. Sci. 1994;35:172-177.
- Healy W. E and Wilkins H. F. Alstroemeria show promise as energy-efficient crop. 1981;(16):40-45.
- Kristiansen K., Ørnstrup H., Brandt B. In vitro PPFD and media composition affect both in and ex vitro performance of Alstroemeria Butterfly-hybrids. Plant Cell Tiss. Org. Cult. 1999;56:145-153.
CrossRef - Santos P. E., Peralta M. C. L.,Hernandez V. A. G., Clark E. M. E and Garcia S P. Plant Cell Tiss. Org. Cult. 2005;84:189-198.
- Pierik R. L., Voorst A., Boody G., Acker C. A. M., Lelivelt C. L. C., Wit J. C. Vegetative propagation of Alstroemeria Hybrids in vitro. Acta. Hortic. 1997;226:143-147.

This work is licensed under a Creative Commons Attribution 4.0 International License.





