How to Cite | Publication History | PlumX Article Matrix
In vitro Regeneration of Alstroemeria cv. ‘Balance’ by Indirect Organogenesis
Hossein Nazarian1, Maryam Beigi Harchegani1, Mahmoud Otroshy2 and Ali Motamedi3
1Department of Agricultural Biotechnology, Payame Noor University, Karaj, I. R. Iran.
2Department of Tissue Culture, Agricultural Biotechnology Research Institute, Najaf Abad, Isfahan, Iran.
3Faculty of Agriculture, Shahid Beheshti University, Tehran, Iran.
Corresponding Author Email: alimotamedi1987@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2484
ABSTRACT: This study was designed in order to optimize the indirect organogenesis (during callus induction and regeneration) of Alstroemeria cv. ‘Balance’ through tissue culture technique in two phases; the first stage: callus induction by rhizome segments, leaf and nodal stem which in the start, callus formation media were examined using two types of auxins; 2,4-D and NAA and a cytokinin; BAP in four different experimentations. In the second stage, calli derived from rhizome segments and nodal stem explants were transferred to regeneration media. The results revealed that 2,4-D in combination with BAP in the rhizome segments and nodal stem explants were efficient as compared to NAA. The highest yield of callus formation was also obtained in the rhizome segments explants. According to the results, it can be suggested that NAA as auxin, does not have direct positive effect on cell division in Alstroemeria. The 2,4-D is toxic at high concentrations and may bring about cell death. Eventually, the composition of 0.5 mg/l NAA with 3 mg/l BAP and callus derived from nodal stem explants may be introduced as the best combination for regeneration. These results indicate the necessity of the BAP cytokinin presence for regeneration. In addition, the maximum length of the shoot was obtained from combination of BAP with nodal stem explants, without the presence of NAA.
KEYWORDS: Alstroemeria; callus induction; organogenesis; PGRs; regeneration
Download this article as:| Copy the following to cite this article: Nazarian H, Harchegani M. B, Otroshy M, Motamedi A. In vitro Regeneration of Alstroemeria cv. ‘Balance’ by Indirect Organogenesis. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Nazarian H, Harchegani M. B, Otroshy M, Motamedi A. In vitro Regeneration of Alstroemeria cv. ‘Balance’ by Indirect Organogenesis. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=25854 |
Introduction
Alstroemeria is classified as a monocotyledon plant from Alstroemeriaceae family (khaleghi et al., 2008). Alstroemeria hybrids are mainly cultured to produce cut flowers in greenhouses (Van Zaayen, 1995). This plant is propagated vegetatively through rhizome splitting (Healy, W.E. and H.F. Wilkins, 1981). This kind of propagation is long-drown-out and lead to the spread of viral contamination. Therefore, in vitro propagation has been extended to accelerate the multiplication efficiency (Gabryszewska and Hempel, 1985;Hakkaart and Versluijs, 1988; VenZaayen et al., 1992; Bond and Alderson, 1993). In the ornamental plants field, tissue culture has permitted mass propagation of superior genotypes and plant improvement, thus enabling the commercialization of healthy and uniform planting material (Nhut, et al., 2006). The success of the micropropagation procedures hinges on various factors such as genotype, media, plant growth regulators and type of explants (Lin and Jacobsen, 2000; pati et al., 2005). Tissue culture and micropropagation are the most important techniques used for rapid in vitro asexual multiplication. Tissue culture technology has a wide range of techniques with high potentials which, in terms of time and space requirements compared to traditional proliferation methods, has economic superiority and provides disease-free plants. However, the requirements for rapid, early returns and independent from genotype are still of concerns about eugenics in Alstroemeria. Callus marks an important source for indirect plant organogenesis and embryogenesis (chato et al., 2006). In Alstroemeria, embryogenic callus was attained from such tissues as zygotic embryos, ovule, seedling tissue and ovary (Khaleghi et al., 2008). According to Seyyedyousefi et al. (2013), node was better explant than internode to produce callus and 0.5 mg/l of BAP and 2.0 mg/l of NAA which induced more callus on the explants. Callus induction is demanding and time consuming in many monocotyledons such as Alstroemeria. Micropropagation implementation and improvement in vitro tissue culture through producing callus is desirable. Thus, the present study aimed at achieving the best explants and plant growth regulators for callus production in Alstroemeria cv. ‘Balance’. The effects of different concentrations of NAA, 2.4-D, BAP and kind of explants on callus formation and regeneration of Alstroemeria, were also investigated.
Materials and Methods
In order to accomplish this research, Alstroemeria cv. ‘Balance’ was collected from greenhouse and used as explants. Explants were washed in sterile, distilled water after each step of disinfection and following the last stage of sterilization, were rinsed three times in sterile, distilled water. After being washed thoroughly under running tap water for 20 min, Rhizome segments (1 cm long) were cut by scalpel and having been through disinfectant treatments, the explants were placed upright in culture media. Having been removed from plants, the young leaves were thoroughly washed under running tap water for 20 min and leave segments (1 cm × 1 cm) were excised by scalpel and following disinfectant treatments, were cultured horizontally in culture mediums. After removal of the young stems of plants which were washed thoroughly under running tap water for 20 min, the upper and lower parts of the stem nodes were cut by scalpel into pieces with a length of 1 cm and the explants were placed in culture mediums vertically. In order to disinfect leaf and nodal stem explants,70% Ethylic alcoholand3.5%sodium hypochlorite were applied for 30 seconds and 15 minutes, respectively. In order to disinfect rhizome segments, 70% Ethylic alcohol and20%sodium hypochlorite were usedfor 1 minuteand35 minutes, respectively. Due to evaluation of callus formation, MS basal medium with four various supplemented composition comprising different levels of plant growth regulators were tested, which included:
First experimentation: NAA concentrations (0, 0.5, 1, 1.5, 2 mg/l)
Second experimentation: BAP concentrations (0, 1, 1.5, 2, 2.5 mg/l)
Third experimentation: NAA concentrations (0, 0.5, 1, 2 mg/l) with BAP concentrations of (0, 0.2, 0.3, 0.5, 1, 1.5, 2 mg/l)
Fourth experimentation: 2,4-D concentrations (0, 3, 9, 12 mg/l) with BAP concentrations of (0, 1, 2, 3 mg/l). In this experiment, the explants subcultures were conducted every two weeks with the same combination of plant growth regulators. After callus induction, the obtained calli from the fourth experimentation of the callus induction test were transferred to MS basal media with different PGRs combinations (to produce buds and to assess regeneration). Organogenesis was evaluated in a separate test. The concentration levels of used plant growth regulators were as follow;
BAP concentrations (0, 1, 2, 3 mg/l) with NAA concentrations (0, 0.5 mg/l)
After preparation of callus induction and regeneration mediums, the media were adjusted at pH 5.8 and solidified with 7 Agar-agar, before autoclaving at 121°C for 20 min.
Environmental Conditions
Each culture jar contained 4 explants (Nodal stem, leaf and rhizome segments). At the end of culture, glasses in the form of closed door were incubated in growth chamber whose environmental conditions were adjusted at 22±2°C and photoperiod of 16 h light to 8 h dark per day. After 90 days, callus formation percentage, the average of callus relative weight (mg), and the best callus weight in culture jar (mg) were measured as assessment parameters. Obtained calli (two calluses placed in every 250 ml jars) from callus formation experiment during a month incubated in growth chamber whose environmental conditions were adjusted to 22±2°C and photoperiod of 16 h light to 8 h dark per day. Then percentage of regeneration, number of regenerated shoots, and shoot length were measured as assessment parameters. These experiments (callus induction and regeneration) were implemented in a factorial design with 3 factors (4 various levels of two different PGRs and origin of explants at 2 levels for callus induction and, respectively, 2 and 4 various levels of two different PGRs and origin of explants at 2 levels for regeneration) with six replications. The obtained data were standardized utilizing “Subtract Mean and Divide by Standard Diviation” in Minitab software version 16.2. Analysis of variance (ANOVA) was done using Duncan’s mean comparison at the probability levels of 0.05, and 0.01by the SAS software version 9.2.
Results
Due to the lack of proper response in the first experiment of direct organogenesis, analysis of variance was not needed. The experiments were followed using nodal stem and rhizome segments, owing to the absence of leaf explants reaction to callus induction by three months and because of chlorosis, senescence and degeneration of explants, which could be attributed to secretion endogenous and exogenous ethylene.
Fourth Experimentation (2,4-D + BAP)
Based on the results of analysis of variance shown in Table 1, the separate and combined effects of 2,4-D and BAP had significant effect on the callus formation (P≤0.01). Among the different levels of 2.4-D, the concentration of 9 mg/l ranked statistically as category ‘a‘(51.63%). In addition, except control, the highest concentration of 2,4-D (12 mg/l), had minimal effect on callus formation. Among the different levels of BAP, the concentration of 3 mg/l had the highest effect on callus formation (36.45%). Concentration of 1 mg/l BAP ranked statistically as category ‘c‘ (16.14%). According to Table 2, concentration of 9 mg/l 2,4-D in combination with the 3 mg/l BAP, was the best treatment regardless with the type of explants (85% callus formation).
According to the analysis of variance (Table 1), the effect of 2,4-D and BAP Individually and also in combination were found significant on the average of callus relative weight (P≤0.01). Results of the comparison of the mean showed that from amongst the different levels of 2,4-D, the concentration of 9 mg/l had the greatest impact on the average of callus relative weight(898.02 mg). In addition, except control, the highest concentration of 2,4-D (12 mg/l), had the minimal effect on the average of callus relative weight. Amongst the different levels of BAP, the concentration of 3 mg/l ranked statistically as category ‘ a'(635.17 mg). Also in the concentration of 9 mg/l 2, 4-D in combination with the 3 mg/l BAP, the highest average of callus relative weight was obtained (1718.50 mg).
Regarding to the analysis of variance indicated in Table 1, the effect of 2,4-D and BAP had a significant effect on the best callus weight in culture jar individually(P≤0.01), but the interaction of the same PGRs were not found to be significant. The results revealed that among different levels of 2.4-D, the concentration of 9 mg/l had the greatest impact on the best callus weight in culture jar. Moreover, save for control, the highest concentration of 2,4-D (12 mg/l) had the minimum effect on the best callus weight in culture jar, which magnifies inhibiting effect of 2,4-D in high concentrations. Considering different levels of BAP, the concentration of 3 mg/l had the most effect on the best callus weight in culture jar.
Based on the obtained results(Table 1), effect of explants, on its own, and the interaction of explants and BAP, also the interaction of explants with the composition of PGRs were not significant on callus formation percentage, the average of callus relative weight (mg) and the bestcallus weight in culture jar (mg), but the combination of explants and 2,4-D,was found to have significant effects on the average of callus relative weight (P≤0.05).Concentration of 9 mg/l 2,4-D in combination with 3 mg/l BAP resulted in the highest percentage of callus formation (87.5%) and the greatest average of relative callus weight (1790.20 mg) in rhizome segments explants, under the condition of every two weeks subculture with the same concentrations of PGRs. The results of the present study indicated that rhizome segments were better explants in callus induction and nodal stem explants had the highest yield of the best callus weight in culture jar.
Table 1: Variance analysis of 2,4-D, BAP and Explants’ effects on the measured parameters in callus formation
| Source | df | callus formation percentage% | the average of callus relative weight (mg) | the best callus weight in culture jar (mg) |
| 2,4-D | 3 | 77.44** | 56.23** | 28.18** |
| BAP | 3 | 87.15** | 51.69** | 98.07** |
| Explant | 1 | 03.51ns | 1.49ns | 00.08ns |
| 2,4-D*BAP | 9 | 23.36** | 80.30** | 01.87ns |
| 2,4-D*Explant | 3 | 10.93ns | 28.15* | 02.62ns |
| BAP*Explat | 3 | 07.92ns | 02.63ns | 01.64ns |
| 2,4-D*BAP*Explant | 9 | 3.28ns | 01.69ns | 01.43ns |
| Error | 153 | 7.7 | 4.69 | 1.31 |
| CV% | 20.27 | 17.89 | 10.61 |
**significantly different at 1% level of probability/ ns; Not significant
*significantly different at 5% level of probability
Second stage; Regeneration
Results of the comparison of the mean (Table 3) revealed that treatments of NAA and BAP PGRs, each separately and the interaction of NAA and BAP, had a significant effect on regeneration (P≤0.01). Considering the various levels of NAA, the concentration of 0.5 mg/l had the most effect on the percentage of regeneration (41.30 %). Taking the different levels of BAP into account, the concentration of 3 mg/l had the maximum impact on regeneration (48.95%). According to the interaction of PGRs treatments, concentration of 0.5 mg/l NAA in combination with 3 mg/l BAP were known as the best condition, or the best treatment regardless to the type of explants (Table 4). In these concentrations, 75% regeneration was observed. Also control ranked statistically as category ‘e’.
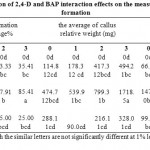 |
Table 2: Means comparison of 2,4-D and BAP interaction effects on the measured parameters in callus formation
|
In each column, means with the similar letters are not significantly different at 1% level of probability using Duncan’s test
Table 3: Variance Analysis of NAA, BAP and Explants’ effects on the measured parameters regeneration
| Source | df | percentage of regeneration % | number of regenerated shoots | shoot length |
| NAA | 1 | 442.43** | 475.22** | 232.72** |
| BAP | 3 | 231.31** | 238.44** | 179.15** |
| Explant | 1 | 69.44** | 69.24** | 46.59** |
| NAA *BAP | 3 | 62.69** | 67.67** | 47.04** |
| NAA *Explant | 1 | 36.90ns | 36.74ns | 14.93ns |
| BAP*Explant | 3 | 30.25ns | 09.63ns | 07.77ns |
| NAA *BAP*Explant | 3 | 11.53ns | 10.30ns | 02.92ns |
| Error | 73 | 5.48 | 5.26 | 3.04 |
| CV% | 16.93 | 16.5 | 13.34 |
**significantly different at 1% level of probability ns; Not significant
*significantly different at 5% level of probability
According to the comparison of the mean (Table 3) the treatments of NAA and BAP PGRs, each separately and the interaction of NAA and BAP, had significant effect on the number of regenerated shoots (P≤0.01). Among the various levels of NAA, the concentration of 0.5 mg/l had the highest effect on number of regenerated shoots (1.69 per explant). Regarding the different levels of BAP, the concentration of 3 mg/l had the maximum impact on the number of regenerated shoots (2 per explant). In concentration of 0.5 mg/l NAA combination with 3 mg/l BAP, the highest number of shoots was obtained (3.08per explant). And control as well as 0.5 mg/l NAA, without the presence of BAP, ranked statistically as category ‘e’ (Table 4).
As shown in Table 3, the effects of NAA and BAP PGRs, each separately and the interaction of NAA and BAP, were found to be significant on the number of regenerated shoot length (P≤0.01). Among the various levels of NAA, the concentration of 0.5 mg/l had the most effect on shoot length (15.01). Taking the different levels of BAP in to consideration, the concentration of 3 mg/l had the maximum impact on shoot length (16.50). Based on Table 4, the highest shoot length was obtained with concentration of 0.5 mg/l NAA in combination with 3 mg/l BAP (19.87 mm). And control ranked statistically as category ‘e’. Pedraza et al. (2006) studied about in vitro regeneration of Alstroeneria cv. ‘Yellow king’ using explants sources by leaf, stem apices, rhizomes and immature inflorescence apices and greatest rate of shoots propagation was obtained on a liquid MS medium at full strength supplemented only with BA at 1 mg/l.
Table 4: Means comparison of NAA and BAP interaction effects on the measured parameters in regeneration
| percentage of regeneration % | number of regenerated shoots | shoot length | |||||||||||
| NAA (mg/L) | 0 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| 0.5 | 0e | 4.16de | 16.6cde | 22.91cd | 0d | 0.16cd | 0.66cd | 0.93bc | 0d | 0d | 11.36cd | 13.14bc | |
| 0e | 15.30c | 52.54b | 75a | 0d | 1.5b | 2.36a | 3.08a | 0d | 14.06b | 15.06b | 19.87a | ||
In each column, means with the similar letters are not significantly different at 1% level of probability using Duncan’s test
As for the comparison of the means (Table 3), treatments of PGRs (NAA and BAP) and explants indicate, each separately was found to be significant (P≤0.01) on evaluate parameters in the regeneration (the percentage of regeneration, the number of regenerated shoots and shoot length). Interaction of each PGR and explants and also the interaction of the NAA, BAP and type of explants, were not significant for evaluated parameters (Table 3). According to Fig.2, among the derived calli from the explants under study, the callus obtained from the nodal stem had accounted the highest percentage of regeneration (31.25%), the highest number of regenerated shoots (1.26 per explant) and the largest shoot length (13.80 mm). In other words, nodal stem seems to be the best of explants during regeneration process (Fig.2). The combination of 3 mg/ l BAP with 0.5 mg/l NAA and nodal stem explants, resulted in increased regeneration (87.5%) (due to the positive efficiency of NAA PGR on regeneration and because of its interaction with BAP). The greatest number of shoot was achieved through the same PGRs and origin of explants (3.5 per explant). The highest shoot length was also obtained through the combination of 3 mg/l BAP with nodal stem explants, without presence of NAA.
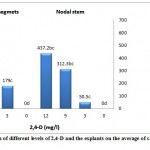 |
Figure 1: Interaction of different levels of 2,4-D and the explants on the average of callus relative weight (mg)
|
Means with the similar letters are not significantly different at 5% level of probability using Duncan’s test
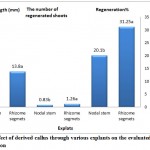 |
Figure 2: Effect of derived callus through various explants on the evaluated parameters in regeneration
|
Means with the similar letters are not significantly different at 1% level of probability using Duncan’s test
Discussion
Callus Formation
The best medium for callus formation or the highest percentage of callus formation is of very high importance, because high percentage of callus formation could make a significant impact on increasing the percentage of regeneration in the next step. Significant descend of callus formation in the concentration of 12 mg/l could be attributed to toxicity of 2,4-D in high concentrations as well as its effects on cell death. Due to the non-efficiency of NAA in combination with BAP and given proof of positive effect of BAP on the callus induction in the present study, it could be concluded that NAA auxin does not have direct positive effect on cell division in the Alstroemeria. Seyyedyousefi et al. (2013) also have noted increasing effect of BAP on callus formation. The results of this phase indicated that the concentration of 9 mg/l 2, 4-D and 3 mg/l concentration of BAP, was the best combination for the highest yield of callus formation. It can be suggested that the lack of response of different levels of BAP (0, 1, 2, 3 mg/l) to callus formation, could be due to the critical role of the presence of auxin (2, 4-D). This is consistent with Khaleghi et al. (2008) who reported the significance of presence of auxin (2, 4-D, NAA) in Somatic embryogenesis in callus induction. Induction of callus from tissues of vegetative plant organs is difficult in monocots (Lin and Jacobsen, 2000). As yet no reports have been published about callus induction in Alstroemeria through 2, 4-D PGR in combination with BAP. The utilized concentrations of PGRs were also novel. Kim et al. (2006) also reported the induction of callus in Alstroemeria from nodal segments. Lin and Jacobsen (2000) reported the induction of callus from stem segments of Alstroemeria. Hormonal balance regulation of auxin and cytokinin is a key factor in the control of cell division in tissue culture (Seyyedyousefi et al., 2013). Reddy et al. (2011) obtained callus from leaf explants when placed on half strength MS media with 2.0 mg/L BAP and 0.5 mg/L 2, 4-D.
Regeneration
The importance of the best medium for regeneration or greatest percentage of regeneration is due to this aspect that high percentage of regeneration can cover low effect of callus formation. Based on the current study results, the presence of NAA PGR is essential in the regeneration and its positive effect and interactions with BAP will lead to increased regeneration. In control and in the culture medium contains 0.5 mg/l NAA, without composition with BAP, no regeneration was induced. These results revealed the necessity of BAP presence for regeneration which accorded with the reports by Lin et al. (1997); Kim et al. (2006); and Pedraza et al. (2006). Based on the present study results, appropriate concentrations of BAP (3 mg/l) with NAA presence in low levels (0.5 mg/l), had a more suitable response to regeneration. Similar results were reported by Han et al. (1994).Thus far, various studies about different varieties of Alstroemeria have been implemented on regeneration. However, no reports have been available on regeneration through derived callus from vegetative plant organs. Khaleghi et al. (2008) evaluated propagation of Alstroemeria cv. ‘Fuego’ through the lateral and terminal buds of rhizomes. Hamidoghli et al. (2007) also studied regeneration of Alstroemeria through rhizome explants derived in vitro and pot plants.
Conclusions
In the studies so far implemented on the tissue culture of Alstroemeria through the indirect organogenesis technique, the regeneration by callus obtained from nodal stem and rhizome segments as explants sources has not been induced. No reports, has ever been available on tissue culture of Alstroemeria cv. Balance. Some merits of this study include: the second stage of indirect organogenesis experiment; regeneration by induced calli from first stage; callus formation by aforementioned explants. No noteworthy results were observed on the utilized PGRs levels in the second stage experiments; regeneration through NAA (0, 0.5 mg/l) and BAP (0, 1, 2, 3 mg/l). Yet other results of the present research include absence of NAA response in the third experimentation of first stage, in combination with BAP (at least in the utilized levels of PGRs) and also non efficiency of these two PGRs, separately, in the first and second experiments (at least in the utilized levels of PGRs) on callus formationــat least in the evaluated genotype.
Acknowledgements
This research was partially supported by Payame Noor University of Iran. We thank our colleagues from biotechnology laboratory that provided insight and expertise that greatly assisted the research, although they may not agree with all of the interpretations of this paper.
Conflict of Interest
All other authors report no conflicts of interest relevant to this article and the conflicts.
References
- Bond S & Alderson P. G. Establishment and growth of the rhizome of Alstroemeria as affected by temperature and the root system. Hortic. Sci. 1993;68:847–853.
CrossRef - Gabryszewska E & Hempel M. The influence of cytokinins and auxins on Alstroemeria in tissue culture. ActaHortic. 1985;167:295–300.
CrossRef - Hakkaart F. A & Versluijs, J. M. A. Virus elimination by meristem-tip culture from a range of Alstroemeria cultivars. Neth. J. Plant Pathol. 1988;94:49–56.
CrossRef - Hamidoghli Y., Bohloli S., Hatamzadeh A. In vitro propagation of Alstroemeria using rhizome explants derived in vitro and in pot plants. Afr J Biotechnol. 2007;6:2147-2149.
CrossRef - Han B. H., Kim Y. J., Choi J. K. Micropropagation of Alstroemeria through rhizome bud culture. J. Korean Soc. Hortic. Sci. 1994;35:172-177.
- Healy W. E and Wilkins H. F. Alstroemeria show promise as energy-efficient crop. 1981;16:40-45.
- Khaleghi A., Sahraroo A., Rasoulnia I. N., Ataei R. In vitro propagartion of Alstromeria cv. ‘Fuego’. Am-Euras. J Agric Environ Sci. 2008;3:492-497.
- Kim J. B., Raemakers C. J. J. M., Jacobsen E., Visser R. G. F. Efficient somatic embryogenesis from leaves with axil tissue in Alstroemeria. Plant Cell Tiss. Org. Cult. 2006;86:233-238.
CrossRef - Lin H. S., De Jeu M. J., Jacobsen E. Direct shoot regeneration from excised leaf explants of invitro grown seedlings of Alstroemeria L. Plant Cell Rep. 1997;16:770-774.
CrossRef - Lin H. S., De Jeu M. J., Jacobsen E. The application of leafy explant micropropagation protocol in enhancing the multiplication efficiency of Alstroemeria. Scientia Horticulturae. 2000;85:307-318.
CrossRef - Nhut D. T., Don N. T., Vu N. H., Thien N. Q., Thuy D. T. T., Duy N., da Silva J. A. T. Floriculture Ornamental and Plant Biotechnology, In: da Silva J. A. T. (Ed), Global Science Books. UK. 2006;325-35.
- Pati P. K., Sharma M., Salar R. K., Sharma A., Gupta A. P., Singh B. Studies on leaf spot disease of Withaniasomnifera and its impact on secondary metabolites. Indian J Microbiol. 2005;48:432–437.
CrossRef - Pedraza-Santos M. E., López-Peralta M. C., González-Hernández V. A., Engleman-Clark E. M., Sánchez-García P. In vitro regeneration of Alstroemeria cv. ‘Yellow King’ by direct organogenesis. Plant Cell, Tissue and Organ Culture. 2006;84:189-198.
CrossRef - Reddy J. H., Bopaiah A. K., Abhilash M. In vitro micropropagation of Anthuriumdigitatum, using leaf as explants. Asian Journal of Pharmaceutical and Health Science. 2011;1:70-74.
- Seyyedyousefi S. R., Kaviani B., Padasht-Dekhaei N., Salehzadeh A. Callus induction in Alstroemeria using NAA and BAP. Pelagia Research Library. European Journal of Experimental Biology. 2013;3(5):137-140.
- Te-Chato S., Susanon T., Sontikun Y. 20 medium influencing embryogenesis and organogenesis in Anthurium cultivar, explants type and culture. Songklanakarin. J. Sci. Technol. 2006;28(4):717-722.
- Van Zaayen A., Loebenstein G., Lawson R. H., Brunt A. A. Alstroemeria. Virus and Virus-like Diseases of Bulbs and Flower Crops, Wiley Publishers, Chichester, UK. 1995;237-249.
- Van Zaayen C., Van E., Versluijs J. M. A. Production of high quality, healthy ornamental crops through meristem culture. Acta Bot. Neerl. 1992;41:425-433.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





