How to Cite | Publication History | PlumX Article Matrix
Behnam Younesi and Mahnaz Azarnia
Department of animal biology, faculty of biological sciences, university of kharazmi, Tehran, Iran.
Corresponding Author E-mail: behnamyounesi94@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2497
ABSTRACT: Oligodendrocytes are types of cells in central neural system (CNS). Their main function is generation of Myelin sheath in CNS, this sheath insulates the Axons. Any disorder in the function of these cells leads to demyelination of neurons and causes neural disorders including multiple sclerosis (MS). Nowadays, cell therapy provides plenty of hope for cure of MS. So far it has used different sources such as stem cells or progenitor for cell therapy of neural system. But each of them had some limitations, for instance using neural stem cells requires certain amount of CNS tissue. Embryonic stem cells also introduced as another candidate for cell therapy but due to some moral problems, such as necessity to creating a Blastocyst, using these cells accompanied many limitations. In cell therapy, the most important factor is facility to acquiring stem cells. iPS cells are kinds of Induced Pluripotent Stem cells which directly created by transferring of 4 transcription factors: oct4, sox2, klf2, and c-Myc into the differentiated cells. iPS cells are like pluripotent embryonic stem cells although they do not require demolition of Blastocyte. Endometrial Stromal cells are kinds of mesenchyme or adult cells which have been proven in human and mice’s uterine endometrial and they are easy to access. Both of these types of cells can be appropriate candidates for cell therapy. In this research we use these two types of cells for differentiate to Oligodendrocytes and we are able to differentiate iPS cells which are from human's eye and also human Endometrial Stromal cells to pre-Oligodendrocytes. Also we can compare their differentiation ability. These cells can be used for transplanting in MS patients.
KEYWORDS: Cell therapy; Endometrial Stromal cell (EnSC) iPS; pre-oligodendrocyte cells; Stem cells;
Download this article as:| Copy the following to cite this article: Younesi B, Azarnia M.Investigating Differentiation Ability of Induced Pluripotent Stem (Ips) Cell and Endometrial Stromal Cells (Enscs) Toward Pre-Oligodendrocytes using Growth Factors In Vitro. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Younesi B, Azarnia M.Investigating Differentiation Ability of Induced Pluripotent Stem (Ips) Cell and Endometrial Stromal Cells (Enscs) Toward Pre-Oligodendrocytes using Growth Factors In Vitro. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=23916 |
Introduction
MS disease is a neural chronic and inflammable disease which causes disorders in function of central neural system. In this disease, Oligodendrocyte cells which myelinate the nerve fibers in neural system affected with defection in their function. Today, the main reason of MS disease is unknown; therefore there is no affective remedy. Up to now, drug therapy has been the main remedy for this disease, but one of the main problems in cure of this disease is temporary remedy of the symptoms and only preventing the progress of the disease. One of the solutions for cure of MS disease is cell transplant and replacement of active Oligodendrocyte in the place of defective one in the patients. The positive results of cell-replacement remedies in transplant of immature neural cells in MS disease and in empirical animal models are reported (Uccelli et al. 2010). Limitation in access to the early Embryonic cells , in accompany culturing and screening of progenitor cells in adult’s brain, which can differentiate neurons and Glias, resulting pay more attention to finding a better cell source, for vast function in cell therapy CNS. It has showed that different stem cells such as mesenchyme stem cell (MSC), Hematopoietic stem cell (HSC), adult’s multi-potent progenitor stem cell (MAPC), Umbilical Cord Blood Stem Cell (UCBSC), Embryonic Stem Cell (ESC) can differentiate to neuron cells (Daily et al. 2005), however these cells encounter to limitations because of unavailability and extract methods and in some cases low proliferation. Elder people’s mesenchyme stem cells do not have a proper function in culture because of the limit capacity (Chow et al. 2009). Therefore in this way our goal in the current study is to consider the differentiation ability of induced Pluripotent Stem Cell (iPSC) and Endometrial Stromal (EnSCs) toward pre-Oligodendrocyte cells using growth factors in vitro.
Cure by Replacing the Cell
Cell therapy means reversion of the missed functions of a cell by replacing a healthy cell in the place patient one. For this purpose, we need stem cells that in culture (in vitro) and in exposure of certain factors we can differentiate them to the special cells. In cell therapy there are two views: one of them is use of adult stem cells from the same tissue, the other one is use of Embryonic stem cells (ESCs), but the usage of these two views has special problems (Liao et al. 2009). In general, a stem cell is a kind of cell that has the ability of self-renewal and also can produce other types of cells. Self-renewal means proliferation and protection of genesis ability for differentiation to other types of cells. Stem cells are in different stages of genesis, so show different degrees of self-renewal and differentiation (Baharvand, 2012). Stem cells have the potential for conveying to other types of the differentiated cells and act as a corrective system for the body, they can divide indefinitely until they can fill in the place of injured cells when the creature is still alive (Mattahai et al., 2009). Stem cells have two important features making them discriminable from the other cells:
Ability to divide and product cells with the same features (self-renewal).
Creating the types of differentiated cells (Colter et al. 2001).
Base on the differentiation ability and plasticity of the stem cells, it can divide them to the following items:
Totipotent Stem Cells
The obtained cells from combination of ovum with spermatozoon and the derived cells after the first dividing of sperm, are fecundated and can make all of the cells such as person’s cells and placental expulsion, like Blastomers of a two-cell embryo that each cell of it can make a complete person.
Pluripotent Stem Cells
The stem cells are weaker than the totipotent stem cells and they can differentiate to the derived cells from every embryonic 3 layers and they have the ability to make the most or all of the person’s cells, but they cannot make placental expulsion. The cells which gained from fetus gonads and they are called embryonic germ cells and the Embryonic Carcinoma cells: ECC are also pluripotent cells.
Multipotent Stem Cells
They create more limited types of cells (like stem cells that exist in adult tissues) these cells can differentiate to some of the cellular lineages which are related to a cellular level.
For instance: hematopoietic stem cell which differentiate to red blood cells, white blood cells and plaquettes (Katler et al., 2001; Winston, 2001).
Using the Stem Cells in Cell Therapy
In cell therapy, one view is usage of embryonic stem cells; these cells have two clear characteristics: (Baharvandi, 2012):
Self-renewal
These cells in vitro, in exposure of different factors can differentiate to all types of cell in body.
Therefore, ICM cells and primordial germ cells are the best source for embryonic stem cells so embryonic stem cells are introduced as appropriate candidates for cell therapy (Glaser et al., 2004). However these cells have plenty of potential, but moral problems that are related to embryonic stem cells consider as a limitation (Chang et al., 2007). So, because of these moral problems, getting embryonic stem cells by Cloning therapy method creates limitations then scientists use the other way for obtaining these cells.
Induced Pluripotent Stem Cells (Ipsc)
Because of the Cloning problems, scientists were trying to activate the genes which were in the early embryonic stages and express them. With progress in gene therapy methods, at first they suggested that the genes which express in the early stages of embryonic in Blastomers and make pluripotent feature in them, enter to a genome of a complete specialized cell by a series of special vectors until it show that whether these cells can reverse to pluripotent status or not. In 2006, Yamanaka and his colleagues analyze and compare the expression of genes in two fibroblastuses cell and embryonic stem cell of mouse, and they introduced 24 candidates of genes for transforming by vector, they consist of:
Ecat 1, Dpp5(Esg1), Fbx015 , Nanog, Eras,Dnmt31,Ecat8, Gdf3,Sox15 , Dppa 2,Fthl17 , Sall4, Oct4, Sox2, Rex1.Utf1, Tcl 1, Dppa3,Klf4, b_cat, cMyc,Stat3,Grb2.
Yamanaka and his colleagues could successfully enter these 24 factors into fibroblastus cells of mouse by retroviruses, after passing of two weeks they observed that these cells conveyed to pluripotent cells which they were similar to embryonic stem cells (ESC) in the way of morphology and growth features, and also they expressed the ES cells marker. These cells are called Induced Pluripotent stem cells or iPS cells. This group of researchers by decreasing these genes in the transfer process finally realized that 4 transcription factors , include: oct4 , c-Myc, Klf2, sox2 are necessary for converting fibroblastus cells to iPSC . Then these researchers inject the iPS cells into a Blastocyst and they observed that these cells contribute in genesis of the fetus. By hypodermic injecting iPSC to the nude mice, it was seen that a cancerous gland in the existed tissues by each of 3 progenitive layers is created (figure 1-1). This wonderful experiment without any moral problems and limitations in cellular source created a new point of view of reprogramming .Somatic cells and pluripotent stem cells in all of cellular populations in morphology, gene expression and functional activities are different, so it is wonderful that just expression of four or even expression of three genes result in this great structure change. It is obvious that all of these transcription factors have specialized and important roles in reprogramming of destination of the differentiated cells (Takahashi et al., 2006). iPS cells similar to embryonic stem cells have all of the pluripotent features and ability of self-renewal and differentiate to different cellular levels stem cells. Using these cells are object point in this way that with availability of the patient’s adult cells, by creating genetic changes in them can get stem cells and by differentiating of these cells we can use them for cell therapy for the same patient (Soldner et al., 2009), furthermore in joining of these cells will not exist some of the immune problems that are result of the incongruent of proteins on the cell surface and this is considered as a new source of the stem cells. These cells can create new hopes in the cell therapy (Ran et al., 2009).
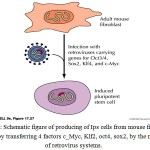 |
Figure 1: Schematic figure of producing of iPS cells from mouse fibroblast cells, by transferring 4 factors c_Myc, Klf2, oct4, sox2, by the means of retrovirus systems. |
Adult Stem Cells
Adult stem cells are multi-potent cells that have more limited differentiation ability than embryonic stem cells and they just can differentiate to more limited cell types. These cells are in many of the specialized tissues of the body such as: brain, marrow, liver, skin, alimentary canal, eye cornea and retina and even Destin pulp (Smith, 2001). In adults when there is injury or there isn’t, these cells are active in a constant manner. Before this scientists thought the adult’s stem cells, create just the cells of its own, but today they believe that these cells plasticity is very much and they can make another types of cells .For example adults’ stem cells that are derived from marrow, in addition to making immune and blood cells, in trans differentiation way they can make another types of cells such as muscular cells, microglia and Astroglia in brain and liver hepatocytes (Herman et al., 2004). These cells separated from different tissues and their differentiating potential may be reflector of their local place, they are lack of special features like ESCs, but in effect of appropriate signals they can differentiate to specialized cells with a differentiated phenotype of early progenitor. Base on the observations it has suggested that maybe these cells can be usable in transplant medicine (Pittenger et al., 1999). In general using adult stem cells in cell therapy with the same tissue encounters to limitation in two aspects:
The source of these cells is limited.
These cells have less ability for proliferating in vitro (Liao et al., 2008).
Recently some rare cells with the same activity of adult stem cells which attract scientists’ attention for using in cell therapy has discovered in human’s womb endometrium.
Mesenchyme Stem Cells or Endometrial Stromal (Enscs) or (Emscs)
Human endometrium or mucous coat of womb has the prominent ability of restoration in woman’s life, and it can go from thickness of 1_0/5mm to 7_5 mm after the menstruation (Mac et al. 1965). Endometrium has the cycling progress of proliferating, differentiation and finally stoppage of activity in menstrual cycle. The growth of functional endometrial in proliferating phase happens in response to increasing of estrogen. Increasing of progesterone in secretory phase causes blockage of epithelial mitosis and start of differentiation of cells. In a situation which nidation of embryo does not happen, metrorrhagia happens and then a new cycle begins, the regeneration happens on the endometrial surface during the metrorrhagia and it completes 24 hours after it (Frenzy et al., 1979). The continuous endometrial growth provides the possibility of pregnancy in people. Since endometrium is a kind of few tissues that exists in an adult which have defluxion and new growing cycle ,existence of stem cells in it and ability of angiogenesis was expressed as a hypothesis since previous years. Recently existence of stromal and epithelial stem cell populations is identified. The presence of stem cells in basal layer of endometrium was introduced since previous years (Report, 2004). Recently, mesenchyme stem cells by simultaneous expressing of two markers PDGFR_β and CD146 has separated from human endometrium. These cells showed their ability in differentiating to Adipocyte, Myoblast, Chondrocyte, Osteoblast in appropriate differentiating places (Schap et al., 2007). The study showed that endometrial stem cells population contains mesenchyme stem cells that are similar to marrow and tooth pulp. Up to now different markers for identifying of endometrial stem cells are used. The oct-4 that is a basic factor for plasticity of stem cells has identified by Immunohistochemistry in human endometrium. The CD117 which is stem cells marker expresses in separated stem cells from menstruation blood. The CD90 is one of the phenotypic markers of mesenchemal stem cells which in accompanying by CD29 and CD105 are used for identifying mesenchyme stem cells from different tissues and endometrium (Tessie et al 2004). Also being enriched of endometrial stem cells from CD146 shows it’s similarity to tooth pulp and marrow stem cells. The endometrial stem cells have showed that they have the ability of angiogenesis. As well as, study on differentiation ability of these cells has showed these cells can differentiate to the cells of every three layers of Endoderm (Hepatocyte), Mesoderm (osteocyte, edipocyte, cardiomyocyte) and Ectoderm (nerve). They are appropriate to replacing with cell therapy. Likewise the researches indicate that these cells after 34 passages still they have a normal Karyotype, proliferation speed of these cells is higher than marrow stem cells (Schap et al., 2005) and most prominent benefit of these cells in proportion to other stem cells is easy access to them.
Pre Oligodendrocyte Cells
Oligodendrocyte progenitors (Ops) after moving in Mammalia CNS put in range with fiber parts of the next white substance then change their forms to pre-oligodendrocyte cells. Pre-oligodendrocyte cells are oligodendrocyte progenitors which are multiprocess and preserve cell division features, have fewer movements and identify by Monoclonal antibody O4. After moving and missing the power of division they alter to immature Oligodendrocyte and finally they differentiate to adult Myelination Oligodendrocytes which express the MAG, MBP and also PLP (Bioman et al. 2005). The production of Oligodenrocyte progenitor cells (OPCs) by the neural stem cells (NSCs) of embryonic periods or Neuroepithelial cells, neonatal periods or neural progenitor and also adultness periods (cell type B) is a result from complex interaction of induce factors and transcription organ in stem cells (Shear et al., 2008).
Research Method
The methods in this research contain experiments for differentiation of two types of stem cells, HEeSCs and iPS cells.
The culture of DMEM/F12, HiPS contains 10 % SR (Serum Replacement) and 10% FBS. This place contains pen/strep with final thickness 50µg/ml, unnecessary amino acids with final thickness 100µM and L Glutamine with final thickness 100mM and Beta mercaptoanol with final thickness 100µM, and 10ng/ml (Baharvand, 2012). The culture of Endometrial Stromal cells and MEF DMEM/F12 have 10 % FBS and pen/strep. The culture of iPS cells was changed every day and the culture of MEF and HEnS every 2 or 3 days. For using MEF as feeder cells of iPS cells, at first it is necessary to stop their proliferation because of metabolic activities, and protect the iPS cells but do not divide. In current study it is performed using Mitomycin C. According to this purpose, 100ul of Mitomycin C (1mg/ml) was dissolve in 10ml culture that was without serum. The solution added to the flask contains MEF cells and we put the flask in incubator for 2_3 hours, it leads to stopping of cell division. After 2 hours we discard the culture contains Mitomycin C and washed 3 times by PBS. After this phase we can cultivate the iPS cells on them.
For each RNA sample, we made cDNA by profiting of Prime Script 1st strand CDNA synthesis kit (The used values were inserted in 6_2 table). The reaction for the kit was 10 minutes in 30 degrees, then 30_60 minutes in 42 degrees and finally 5 minutes in 95 degrees, respectively.
Table 1: The method of CDNA
| The volume | Substance name | Line |
| 1µm
1µm Total RNA:<5µg Poly(A)+RNA:<1µg Up to 10µl |
Oligo Dt primer or Random Hexamer(50um)
Dntps Mixture Template RNA RNase_free water |
1
2 3 4 |
A_ The method of template RNA primer Mixture with Prime Script 1st strand CDNA synthesis Kit.
Table 2: The method of reaction with Prime Script 1ST strands CDNA synthesis Kit
| The volume | Substance name | Line |
| 10µl
4µl 0.5µl 1µl Up to 20µl |
Template RNA primer
5x primer script Buffer Rnase inhibitor Primer scribe RTase RNase_free water |
1
2 3 4 5 |
B_ The method of reaction with Prime Script 1ST strand CDNA synthesis Kit.
Results
The Results of Differentiation of Hens Cells
The analyze of flow cytomeric of construction in which Endometrial stem cells are positive for mesenchyme stem cells markers such as CD44, CD146, CD90, CD105 and pluripotent marker OCT4, but they are negative for Endometrial CD31 cells markers and Hemapoietic CD34 and CD133.The results are mentioned in Fig._2.
 |
Figure 2: Flow cytometric of expression of mesenchyme and non-mesenchyme in endometrial stem cells
|
The Results of Differentiation to Pre_Oligodendrocyts
In current study, at the end of fourth, fifth and sixth phases differentiation of expression of markers in each phases are were considered by a qualitative technic Real time PCR.
It was expected to observing the progenitor neural cells markers (Nestin) in fourth phase and Oligodendrocyt marker SOX10 in fifth and sixth phases. In fourth, fifth, sixth phases the expression of specialized markers on the protein surface were considered by Immunocytochemy method. The investigation of taken photos during the study indicated that after adding FGF2/EGF, morphology of treatment cells were starting to change step by step. The cell superfluities cleared and the cells began elongation. The longest state was seen after adding PDGF in treatment cells. The bipolar Oligodendrocyte-like cells began to be long, when the hormone T3 was added, some of them also appeared as multipolar. The control group cells were unchanged and these cells have kept the undifferentiated cells features (a lot of cytoplasm and superfluities). Accompanying Endometrial cells that differentiated to Oligodendrocite-like cells there were neural cells. Sometimes it is seen the scattered cells have contact by the means of the created superfluities in far distances, so that it is possible 4-5 cells contact sequentially. The outcomes are mentioned in fig. 3.
 |
Figure 3: The photomicrograph of differentiated stem cells to Oligodendrocyte in control environment (a) after adding FGF2/EGF, (b) after GF2/PDFG(c) after adding T3 (d) (Zoom ×100) |
As well as the coloring by specialized neurons and Oligodendrocyte demonstrated these cells after treating with various growth factors, expressed neural marker Nestin and Oligodendrocyte A2B5 and Olig2 markers.
Considering RT_PCR
The PCR considerations are performed in three various stages: after adding FGF2/EGF and FGF2/PDGF and T3. The mRNAs consideration results of neural markers and Oligodendrocyte include Nestin and sox10 indicated they were treated in cells with FGF2/EGF, Nestin was expressed in a high rate but in the next phases this rate reduces gradually.Sox10 that is an important transcription factor for expressing Oligodendrocyte genes had a low expression rate but in progressing phases of differentiation, its expression rate raised. The outcomes are in fig.4.
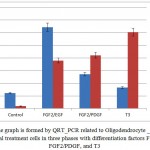 |
Figure 4: The graph is formed by QRT_PCR related to Oligodendrocyte _ like cells of endometrial treatment cells in three phases with differentiation factors FGF2/EGF, FGF2/PDGF, and T3 |
The Consequences from Differentiation of Hips Cells to Pre_Oligodendrocyte Cells
The hiPS cells are useful, pluripotent and feeder dependent cells which observe on feeder layer of MEF cells. At the end of the fifth, sixth and seventh phases, the expression of specialized markers were investigated on the surface of protein by Immunocytochemy method.
At the second differentiation phase, a stage was made for forming of embryiod bodies from HiPS cells in EB structures. The investigating of taken photos during the study indicated the iPS cells when lacks of BFGF were in Teflon dishes and in a place; they showed the ability of EB production. In the embryiod bodies, the desired features for differentiation of embryonic stem cells are spherical appearance and arranged margins, the EB structures had round and arranged margins (fig. 5).
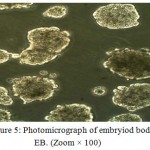 |
Figure 5: Photomicrograph of embryiod bodies EB. (Zoom × 100) |
After adding FGF2/EGF, the treatment EB morphologies began to change gradually. The EB cells started to separating, the superfluities cleared, and the cells began elongation. The longest state was seen after adding PDGF in treatment cells. The bipolar Oligodendrocyte-like cells began to be long, when the hormone T3 was added, some of them also appeared as multipolar but the control group cells were unchanged. Accompanying iPS cells that differentiated to Oligodendrocite-like cells there were neural cells. Sometimes it is seen the scattered cells have contacts by the means of the created superfluities in far distances, so that it is possible 4-5 cells contact sequentially. The outcomes are mentioned in fig. 6.
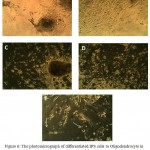 |
Figure 6: The photomicrograph of differentiated IPS cells to Oligodendrocyte in control environment (A) and after adding FGF2/EGF, (B) after FGF2/PDFG (C, D) and after adding T3, (E) (zoom×100) |
The coloring by specialized markers of progenitor neural cells and Oligodendrocyte progenitors demonstrated these cells after treating with various growth factors, expressed progenitor neural marker Nestin and Oligodendrocyte A2B5 and Olig2 markers. The outcomes of Immunocytochemy investigations are in fig.7.
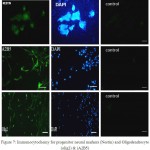 |
Figure 7: Immunocytochemy for progenitor neural markers (Nestin) and Oligodendrocyte (olig2) & (A2B5) |
In general in current study we used expressing investigation of genes in MRNA level and Real Time-PCR method and neural markers of Immunocytochemy and Oligodendrocyte for identifying the differentiation of neural progenitors and Oligodendrocyte. According to the performed investigations by the means of mentioned methods in these differentiated cells and comparing them to control group, we achieved this outcome that Endometrial stem cells have the ability of differentiating to Oligodendrocyte progenitors.
Conclusion
The considering of the outcomes indicates that endometrial adult stem cells and induced pluripotent stem cells which are derived from human’s eye can differentiate to Oligodendrocyte progenitors when they put in the neural cultures accompanying growth factors. The hiPSC and HEnSCs are appropriate replacements for restoring of neural system because they have important and potential advantages. The access to HEnSCs is simpler and more available than other stem cells. Producing of iPS cells requires difficult stages like transferring of gen; unfortunately one of these genes is an oncogene that is called c-Myc and cause cancer. Delivery method of gen is an important subject for producing of iPS cells. For being sure that pluripotent factor expressions are performed, the virus vectors should be used. It is necessary to silence the expression of transferred factors in following successful reprogramming. Since some of the required pluripotent factors are oncogics. The viruses that interpose the transferring of the gene are general methods for producing of iPS cells. Using them cause a problem because entrance of the viruses can break the genes in entrance place. In addition, the entrance of an active transcription virus genome to adjacency of interior gene leads to activating of transcription (and it is possible to silencing genes). This is a serious matter because the entrance activity has important results, for example retroviruses that interpose the entrance to Hematopoietic cells can increase the self-renew in vitro which cause cancer in vivo. The EnSCs are available easily and do not need to gene transferring methods for production, they are not pluripotent like iPS cells and they have low differentiation ability. Although in cell therapy the main choice is access to these cells. One of the problems of EnSCs is their limited usage (only in women); this matter causes the increase of human need for using iPS cells in cell therapy.
References
- Baharvand H. Embroynic Stem Cells Biology house publishing the 1th & 3th chapters. 2012;13(21):70_85.
- Schwab K. E & Gargett C. E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Human Reproduction. 2007;22(11):2903-2911.
CrossRef - Ren Z & Zhang Y. Cells therapy for Parkinson’s disease—so close and so far away. Science in China Series C: Life Sciences. 2009;52(7):610-614.
CrossRef - Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D & Marshak D. R. Multilineage potential of adult human mesenchymal stem cells. science. 1999;284(5411):143-147.
- Liao J., Wu Z., Wang Y., Cheng L., Cui C., Gao Y & Dai H. Enhanced efficiency of generating induced pluripotent stem (iPS) cells from human somatic cells by a combination of six transcription factors. Cell research. 2008;18(5):600.
CrossRef - Matthai C., Horvat R., Noe M., Nagele F., Radjabi A., Van Trotsenburg M & Kolbus A. Oct-4 expression in human endometrium. Molecular human reproduction. 2006;12(1):7-10.
CrossRef - Kang S. M., Cho M. S., Seo H., Yoon C. J., Oh S. K., Choi Y. M & Kim D. W. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007;25(2):419-424.
CrossRef - Glaser T., Perez-Bouza A., Klein K & Brüstle O. Generation of purified oligodendrocyte progenitors from embryonic stem cells. The FASEB journal. 2005;19(1):112-114.
CrossRef - Hermann A., Gastl R., Liebau S., Popa M. O., Fiedler J., Boehm B. O & Storch A. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. Journal of cell science. 2004;117(19):4411-4422.
CrossRef - Ferenczy A., Bertrand G & Gelfand M. M. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol. 1979;133(8):859-867.
CrossRef - Daley G. Q., Goodell M. A & Snyder E. Y. Realistic prospects for stem cell therapeutics. ASH Education Program Book. 2003(1):398-418.
CrossRef - Winston R. Embryonic Stem cell research-The case for. Nature medicine. 2001;7(4):396_397.
- Chua S. J., Bielecki R., Wong C. J., Yamanaka N., Rogers I. M & Casper R. F. Neural progenitors neurons and oligodendrocytes from human umbilical cord blood cells in a serum-free, feeder-free cell culture. Biochemical and biophysical research communications. 2009;379(2):217-221.
CrossRef - Colter D. C., Class R., DiGirolamo C. M & Prockop D. J. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proceedings of the National Academy of Sciences. 2000;97(7):3213-3218.
CrossRef - Baumann N & Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiological reviews. 2001;81(2):871-927.
CrossRef - Tsai M. S., Lee J. L., Chang Y. J & Hwang S. M. Isolation of Human Multipotent Mesenchymal Stem Cells from Second‐Trimester amniotic fluid using a novel two‐stage culture protocol. Human reproduction. 2004;19(6):1450-1456.
CrossRef - Uccelli A., Mancardi G. Stem cell transplantation in multiple sclerosis. Curr Opin Neurol. 2010;23:218–225.
CrossRef - Takahashi K & Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. cell. 2006;126(4):663-676.
CrossRef - Smith A. G. Embryo-derived stem cells of mice and men. Annual review of cell and developmental biology. 2001;17(1):435-462.
CrossRef - Soldner F., Hockemeyer D., Beard C., Gao Q., Bell G. W., Cook E. G & Isacson O. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964-977.
CrossRef - Sher F., Balasubramaniyan V., Boddeke E & Copray S. Oligodendrocyte differentiation and implantation: new insights for remyelinating cell therapy. Current opinion in neurology. 2008;21(5):607-614.
CrossRef - Schwab K. E., Chan R. W. S & Gargett C. E. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertility and sterility. 2005;84:1124-1130.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





