How to Cite | Publication History | PlumX Article Matrix
Low Cost Method of Activation of Micro test Plate for Image-Based Diagnostics
Shahila Parween1, Azmi Naqvi2, Dinesh C. Sharma2 and Pradip Nahar3
1Department Innovative Diagnostics, CSIR-Institute of Genomics and Integrative Biology, Mall Road, Delhi – 110 007, India.
2Department of Zoology, Km. Mayawati Government Girls P.G. College, Badalpur, Gautam Buddha Nagar (U.P.) - 203207.
3New Idea Lab, C-202, Ramkrishnan Cooperative Group Housing Society, Plot-12, Sector 23, Dwarka, New Delhi 110077, India.
Corresponding Author E-mail: azminqv@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2504
ABSTRACT: In this communication, we report the activation of micro test plates (polypropylene plate) by introducing an active functional group through photolinker either by artificial UV irradiation or by sunlight exposure for image based diagnostics. This simple and unique technique leads to specificity and sensitivity of the assay. The, sunlight mediated activation of micro test plate can be a potential alternative to artificial UV light, as the required sunlight intensity for activation is available in most parts of the world and also this method is cheap, eco friendly, and is a clean energy source. Optimum activation was obtained at 90,000 lux, beyond this sunlight exposure produced almost uniform activation. Thus, this rapid and simple method of surface activation could be used for the immobilization of different biomolecules such as protein, DNA, or carbohydrate which can be used for different purposes including ELISA and other related techniques on micro test plate.
KEYWORDS: Photolinker; Immobilization; Microtest plate; Immobilization
Download this article as:| Copy the following to cite this article: Parween S, Naqvi A, Sharma D. C, Nahar P. Low Cost Method of Activation of Micro test Plate for Image-Based Diagnostics. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Parween S, Naqvi A, Sharma D. C, Nahar P. Low Cost Method of Activation of Micro test Plate for Image-Based Diagnostics. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=24418 |
Introduction
Immobilization of biomolecules onto an inert polypropylene surface has diverse applications and is of special interest because (i) their insolubility in most of organic solvents and (ii) their inability to bind through adsorption (unlike polystyrene). Moreover, immobilization of biomolecules onto polypropylene surface is not easy as they lack any active functional group for chemical bonding. Therefore, it is necessary to introduce active functional group to this surface for immobilization of biomolecules. Activation of polypropylene surface has been reported to occur through plasma activation1-3 by introducing hydroxyl or amino groups by a plasma technique employing oxygen or anhydrous ammonia.4 All these methods of activation of this surface is tiresome and time consuming, hence, there is a need for development of convenient and straight technique for introduction of active functional groups onto this surface. Nahar et al 5 have published a simple, rapid and mild procedure for light-induced activation of inert polymers. Such photo-activated polymers are very efficient in binding a protein molecule without any additional reagent.6-7 The light induced immobilization technique is mild, biomolecule friendly and independent of pH and temperature and has the potential to immobilize a biomolecule irrespective of its functional group. The same group later published a paper to introduce active functional group to the surface of polypropylene surface.8
Herein, we report activation of polypropylene microtest plate surface PPµTP by FNAB. Here we have studied different parameters for making activated polypropylene microtest plate surface (APPµTP) as (i) Optimization of photolinker viz. 1-fluoro 2 nitro 4 azido benzene (FNAB) concentration, (ii) effect of temperature and (iii) light exposure time. Further we have studied activation of PPµTP by sunlight as an energy source which is cheap and versatile source of energy.
Materials and Methods
Material
Horseradish peroxidase (HRP) and o-phenylene diamine dihydrochloride (OPD), bovine serum albumin (BSA) were purchased from Sigma – Aldrich, USA. Phosphate buffered saline (PBS) was prepared by mixing 0.85% NaCl to 0.01M phosphate buffer (pH 7.2). Polypropylene sheet was purchased locally and microtest plates were prepared as mentioned by Parween and Nahar. 9 Substrate dye buffer was prepared by mixing 12ml of citrate buffer (0.025M citric acid and 0.05M Na2HPO4 · 2H2O, pH 5), 5 µl of H2O2 (30% w/v), and 4mg of o-phenylenediamine.
Experimental Methods
Preparation of fluoro-2-nitro-4-azidobenzene (FNAB)
NaNO2 solution (24 g/ 60 ml of water) was added dropwise to the clear and cooled solution of (50 g) 4-fluoro – 3-nitroaniline which was dissolved in the mixture of 325 ml warm concentrated HCl and 60 ml water and filtered while hot. After this, the reaction mixture was stirred continuously at a temperature of about -20°C. After the addition, of the above reagents the reaction mixture was stirred for additional 15 min followed by dropwise addition of NaN3 (22 g/ 80 ml water) to the reaction mixture. The temperature was maintained at around -20°C. After the addition, it was stirred for another 15 min. Yellow product was formed which was filtered and washed in ice-cold water. The product was recrystallized from light petroleum to give 36.5 g needle-shaped, straw-colored crystals of 1- fluoro-2-nitro-4-azidobenzene (IUPAC: 4-azido-1-fluoro- 2-nitrobenzene), mp. 52°C. FNAB was stored in dark in a loosely capped bottle at 4°C in a refrigerator.
Preparation of APPµTP in a Photochemical Reaction Induced by UV Light
Optimization of Amount of FNAB
Different concentrations of FNAB (1, 0.5, 0.25, 0.125, 0.0625, 0.0312 and 0 mg) were dissolved in 2.5 µl/cavity of methanol and loaded onto the cavities of PPµTP [9]. The plate was kept for 5 min in dark hood for complete evaporation of methanol from the wells. The plates were then exposed to UV light for 20 min at a wavelength of 365 nm in UV Stratalinker fitted with five 15-W tubes. The plate was then washed with methanol and air dried.
Checking The Efficacy of Activation
Horse Radish Peroxidase (HRP) solution (100 ng/10μl of PBS) was added into each cavity of the PPµTP and incubated at 50°C for 45 minutes. The plate was washed with washing buffer (PBS-Tween 20). After washing, substrate – dye buffer (8 μl/ cavity) was added into each cavity and after 5 minutes 2 μl of stop solution was added. The plate was scanned on a desktop scanner. 10 All the experiments were performed in triplicates.
Similar method of HRP immobilization followed by its assay was used to check the efficacy of activation on APPµTP in the other experiments of activation.
Effect of Different UV Exposure time for FNAB Activation
Four PPµTP having three cavities in each plate was made. The cavities of these plates were then loaded with FNAB (0.0625/ 2.5 µl methanol/ cavity). Plates were kept for 10 min in hood for complete evaporation of methanol. The plates were irradiated in an UV strata linker at 365 nm for 10 min, 20 min, 30 min and 40 min respectively. Cavities were washed with methanol and dried.
Solution and Dry Phase Activation of Ppµtp
PPµTP were activated using FNAB (1, 0.5, 0.25, 0.125, 0.0625 and 0.03125) mg / 2.5 µl solvent / cavity by exposing them to UV light for 20 minutes. When methanol was used as a solvent, the plates were air dried in a fume hood and exposed to UV light for 20 min. In case of CCL4; solvent was not evaporated and directly exposed to UV light for 20 min.
Preparation of Appµtp in A Photochemical Reaction Induced by Sunlight
Optimization of Amount of FNAB
Different concentrations of FNAB (1, 0.5, 0.25, 0.125, 0.0625, 0.03125 and 0.015625) mg in 2.5 µl methanol/cavity were loaded into the triplicate cavities of PPµTP in 7 consecutive rows. One row was left untreated (no activation). This was done under dark conditions until the solvent was evaporated completely. Plates were then exposed to sunlight having intensity of 80,000-90,000 for 20 min for activation. After exposure the plates were washed with methanol. Sunlight activation was carried out in Delhi (latitude 28.38 N, longitude 77.12 E), India in winter (temperature 23°C).
Effect of Sunlight Intensity
FNAB (0.0625 mg/2.5 µl methanol/cavity) coated plates were exposed to sunlight for 20 min at 8 am, 9 am, 10 am, 11 am, 12 pm and 1 pm. Sunlight Intensity was measured by lux-meter. One FNAB coated plate was kept in dark for 20 minutes. After activation the plates were washed with methanol.
Effect of Sunlight Exposure Time
FNAB solution (0.0625 mg/2.5 µl methanol/cavity) was poured into cavities of PPµTPs followed by slow evaporation of methanol in dark in a chemical hood. PPµTPs were then exposed to sunlight at an intensity of 80,000-90,000 lux for 10, 15, 20 and 25 min respectively. As a control, one FNAB coated plate was exposed to UV- light for 20 minutes. After light exposure the activated plates were washed with methanol.
Effect of Temperature
FNAB (0.0625 mg/2.5 µl methanol/cavity) coated plates (5 different plate with three cavities each) were exposed to sunlight at an intensity of 87,000-93,000 lux at different temperatures (0ºC, 16ºC, 26ºC, 30ºC, and 40ºC respectively) for 15 minute. Activated plates were washed with methanol and the color of the cavities was observed.
Comparison Between Sunlight and UV-Light Mediated Activation of Ppµtps
Two sets of FNAB (0.0625 mg/2.5 µl methanol/cavity) coated PPµTPs were exposed to sunlight and UV light for 15 and 20 minute respectively at 30ºC, followed by HRP immobilization and color development. The plates were then scanned in desktop scanner and the result was then expressed as saturation percentage.
Covalent Binding of Biomolecules on Appµtp
PPµTP was taken in which only the triplicate cavities of second row was activated. The triplicate cavities of first row were kept untreated. HRP (100 ng/cavity) was immobilized by incubating for 45 minutes at 50ºC. In another plate cavities were immobilized first with BSA (2 mg/ml) followed by HRP immobilization. Substrate dye was added for color development. The plates were then scanned in a desktop scanner to get the image.
Results and Discussion
PPµTP was activated by introducing an active functional group on the inert surface through a photo linker, 1-fluoro-2-nitro-4-azidobenzene in a photochemical reaction. The azido group of FNAB (i) upon UV excitation is transformed into a highly reactive nitrene (ii) which inserts into the C–H bonds of the polymer (iii) by a covalent linkage whereas the active fluoro group of FNAB, now part of the polymer, remains intact. The activated polymer (iv) binds with the protein following displacement of its fluoro group by the amino group of the protein producing an immobilized protein (v) (scheme 1.1). This simple, rapid and one step method of surface activation allows covalent binding of any biomolecule bearing a nucleophillic group.
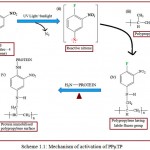 |
Scheme 1.1: Mechanism of activation of PPµTP
|
UV-light Mediated Activation
Activated PPµTP should give maximum immobilization of biomolecules, for this, it was necessary to determine the optimum amount of FNAB required for activation. Maximum activation (more color after enzyme assay) was observed when the amount of FNAB was kept at 0.0625 mg per cavity, beyond which no remarkable difference was seen (Figure 1.1). Volume of solvent used was important and it was found that 2.5 µl per cavity was optimized for such a small test zone. When using 10 µl and 5 µl, FNAB spilled out and was seen lying above the cavities on evaporation. Thus 2.5 µl solvent was optimized volume. Concentrated FNAB beyond 0.5 mg/2.5 µl of methanol /cavity was not desirable due to precipitation of FNAB even before adding to the cavity. Desired solution volume was 2.5 µl /cavity as FNAB did not come out of the cavity during evaporation thus restricting FNAB to remain in the cavity.
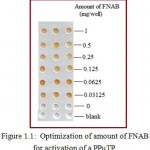 |
Figure 1.1: Optimization of amount of FNAB for activation of a PPµTP.
|
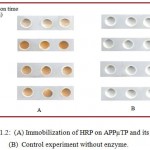 |
Figure 1.2: (A) Immobilization of HRP on APPµTP and its assay. (B) Control experiment without enzyme. |
Figure 1.2 shows optimized UV irradiating time which was found to be 20 min as seen by immobilizing HRP and then observing the color. Even when irradiated for longer time showed approximately same O.D. value. At lower time intervals, there was inadequate surface activation following little enzyme immobilization and thereby low O.D values and less intense color.
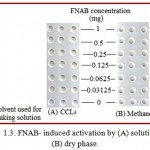 |
Figure 1.3: FNAB- induced activation by (A) solution and (B) dry phase.
|
FNAB induced photo activation of polypropylene surface was carried out using methanol and CCL4. Methanol was considered better than CCL4 as it left no yellowish color as seen when CCL4 was used (Figure 1.3).
Sunlight – Mediated Activation
Amount of FNAB required for the activation of cavity was optimized to be 0.0625 mg /cavity. This concentration gave the maximum immobilization of HRP. Cavities which were not activated by FNAB showed no color (Figure 1.4). This was in good agreement with that of UV-light-mediated activation with the same FNAB concentration.
When sunlight mediated activation of PPµTPs with different exposure time was compared, the plates activated by 15 min sunlight exposure showed higher enzyme immobilization and high color in the cavities. Exposure at 15 min and 20 min both showed similar results whereas exposure at 25 min shows slight yellow color on the surface of the cavities which may hinder in the quantification of color (Figure 1.5). Thus, 15 minutes was optimized time for sunlight mediated activation.
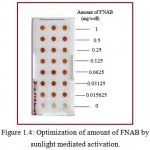 |
Figure 1.4: Optimization of amount of FNAB by sunlight mediated activation.
|
 |
Figure 1.5: Activation of PPµTP under sunlight in a) 10 min, b) 15 min, c) 20 min, and d) 25 minutes. UV mediated activation (e) is treated as positive control. Efficacy of activation was checked by immobilizing HRP followed by its assay.
|
For each experiment one cavity was left without immobilization as negative control.
Optimization of sunlight intensity was very important for the activation of cavities because it varies at every place and also time to time. In order to study the effect of sunlight intensity, FNAB coated plates were kept for 20 min under sunlight at different times of the day. The sunlight intensity varied at different time of a day ranging from 9200 lux in the morning (8 am); 18,700 lux at 9 am; 39,800 at 10 am; 72,000 at 11 am; 89,800 at 12 pm and 99,000 at 1 pm. It was observed that under low sunlight intensity there was less activation as less color was seen after HRP immobilization. Optimum activation was obtained at 90,000 lux, beyond this sunlight exposure produced almost uniform activation. In the dark (0 lux) there was no activation of the PPµTP surface was seen. (Figure 1.6).
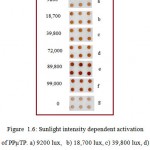 |
Figure 1.6: Sunlight intensity dependent activation of PPµTP. a) 9200 lux, b) 18,700 lux, c) 39,800 lux, d) 72,000 lux, e) 89,800 lux, f) 99,000 lux and g) 0 lux (under dark).
|
The effect of temperature was determined by keeping the FNAB coated cavities at different temperature (0ºC, 16ºC, 26ºC, 30ºC, and 40ºC respectively) under sunlight for 15 minutes. Minimum color was observed on the plates kept at 0ºC. 30ºC was found optimum below which the plates showed less activation and beyond which PPµTPs showed slight yellow color on their cavities (Figure 1.7).
We have also compared UV-light and sunlight mediated activation of PPµTP with respect to their optimized conditions. The color obtained on a sunlight-mediated surface activation (15 minutes, 30ºC having sunlight intensity of 85,000-93,000 lux) was similar to that obtained by UV-light mediated activation (20 minutes, UV-light under 365 nm in UV-Stratalinker 2400) (Figure 1.8).
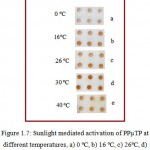 |
Figure 1.7: Sunlight mediated activation of PPµTP at different temperatures, a) 0 ºC, b) 16ºC, c) 26ºC, d) 30ºC and e) 40ºC. Sunlight intensity was maintained at 87,000-93,000 lux.
|
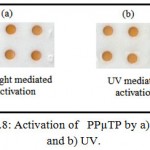 |
Figure 1.8: Activation of PPµTP by a) sunlight and b) UV.
|
To find out whether APPµTP binds covalently or not, we first immobilized BSA onto activated surface prior to immobilization of HRP by ultra sound energy (Scheme 1.2). The cavities showed no color, indicating that HRP immobilization does not occurred; this is expected as the labile fluoro group of the activated surface might have been exhausted by
NH2–group of BSA and no further reactive moieties are available for covalent binding of HRP. On the other hand, cavities where only HRP was immobilized showed good color. Also, untreated surface does not showed any binding of enzyme which suggests that APPµTP shows only specific binding (Figure 1.9).
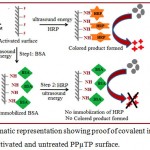 |
Scheme 1.2: Schematic representation showing proof of covalent immobilization of a protein onto an activated and untreated PPµTP surface.
|
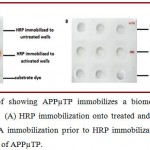 |
Figure 1.9: Proof showing APPµTP immobilizes a biomolecule only through covalent binding. (A) HRP immobilization onto treated and untreated cavities of APPµTP (B) BSA immobilization prior to HRP immobilization onto treated and untreated cavities of APPµTP.
|
Conclusions
Thus, the PPµTP are activated by introducing an active functional group through 1-fluoro-2 nitro-4-azidobenzene either by UV irradiation or sunlight exposure. This simple and unique technique leads to specificity and sensitivity of the assay. The, sunlight mediated activation can be a potential alternative to artificial UV light, as the required sunlight intensity for activation is available in most parts of the world and also this method is cheap, eco friendly, and is a clean energy source. Thus, this rapid and simple method of surface activation could be used for the immobilization of different biomolecules such as protein, DNA, or carbohydrate which can be used for different purposes including ELISA and other related techniques on APPµTP.
Acknowledgment
Authors Declare No Conflict of Interest
A.N thanks the University Grant Commission, Government of India for the award of a “Post Doctoral Fellowship” for Women. S.P. thanks the Council for Scientific and Industrial Research, India for the award of a senior research fellowship
References
- Radić N., Dojčinović B., Obradović M. B., Kuraica M. M., Černák M. Depositon Of Silver On Plasma Activated Polypropylene Surface By Dielectric Barrier. Discharge Astron. Obs. Belgrade . 2010;89:311-314.
- Szabová R., Černáková Ľ., Wolfová M., Černáka M. An Introduction to Plasma Treatment and Its Applications in Surface Modification of Polypropylene. Acta Chimica Slovaca. 2009;2:70 – 76.
- Sipehia R. FTIR-ATR Spectra of Protein a Immobilized on to Functionalized Polypropylene Membranes by Gaseous Plasma of Oxygen and of Anhydrous Ammonia. Biomater Artif Cells Artif Organs. 1989;16(5):955-966.
CrossRef. - Bertrand O. F., Sipehia R., Mongrain R., Rodés J., Tardif J. C., Bilodeau L., Côté G., et al. Biocompatibility aspects of new stent technology. Journal of the American College of Cardiology. 1998;32(3):562-71.
CrossRef. - Nahar P.,Wali N. M and Gandhi R. P. Light-induced activation of an inert surface for covalent immobilization of a protein ligand. Analytical Biochemistry. 2001;294;148-153.
CrossRef. - Bora U., Chugh L., Nahar P. Heat-mediated enzyme-linked immunosorbent assay (HELISA) procedure on a photoactivated surface. J Immunol Methods. 2002;15;268(2):171-7.
- Bora U.,Sharma P., Kannan K., Nahar P. Photoreactive cellulose membrane – A novel matrix for covalent immobilization of biomolecules. J Biotechnol. 1;126(2):220-9.
- Naqvi A., Nahar P., Gandhi R. P. Introduction of functional groups onto PP and PE surfaces for immobilization of enzymes Analytical Biochemistry. 2002;306(1):74-78.
CrossRef. - Parween S., Nahar P. Image-based ELISA on an activated polypropylene microtest plate spectrophotometer-free low cost assay technique. Biosensors and Bioelectronics. 2013;48:287–292.
CrossRef. - Parween S., Nahar P. Femtogram detection of horseradish peroxidase by a common desktop scanner. Journal of Bioscience and Bioengineering. 2015;113-116.
CrossRef.

This work is licensed under a Creative Commons Attribution 4.0 International License.





