Manuscript accepted on : 12 May 2017
Published online on: --
Plagiarism Check: Yes
Shweta Sachan and Aditi Singh
Amity Institute of Biotechnology, Amity University Uttar Pradesh, Lucknow Campus, Malhaur, GomtiNagar Extension, Lucknow India.
Corresponding Author E-mail: asingh3@lko.amity.edu
DOI : http://dx.doi.org/10.13005/bbra/2506
ABSTRACT: A widely employed strategy for increased production is to use crop residues as nutrient source in bioprocess. The plan of present work is to modify growth conditions by using by-products of Azadirachta indica (neem) oil seed cake and optimize environmental conditions for maximum production of extracellular lipases by P. aeruginosa JCM5962(T), identified for production of extracellular lipases previously. Semi solid state fermentation medium comprising of different concentrations of Azadirachta indica oil cake (AIOC) as substrate was studied, in which 4% of AIOC supported maximum lipase production after 96 hours of incubation at 37ºC. Optimization of growth condition was done using nitrogen supplements, carbon additives and various oils. 1% Ammonium nitrate as additional nitrogen supplement was found to be ideal parameter for improved production, whereas carbon supplements and oil additives did not have any effect. Mixed substrates of AIOC with six other oil cake residues (1:1) were also studied. Additive effect of mustard and coconut oil cake caused an increase of 29.95% and 38.26% respectively as opposed to AIOC alone. It is the first report demonstrating use of Azadirachta indica oil seed cake as fermentation medium for improved production of extracellular lipase from Pseudomonas aeruginosa JCM5962(T).
KEYWORDS: Azadirachta indica; Lipase; NH4NO3; Oilcakes; Pseudomonas aeruginosa
Download this article as:| Copy the following to cite this article: Sachan S, Singh A. Production of Lipase by Pseudomonas Aeruginosa JCM5962(T) Under Semi-Solid State Fermentation: Potential use of Azadirachta Indica (Neem) Oil Cake. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Sachan S, Singh A. Production of Lipase by Pseudomonas Aeruginosa JCM5962(T) Under Semi-Solid State Fermentation: Potential use of Azadirachta Indica (Neem) Oil Cake. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=25274 |
Introduction
Oil cakes are by-products, which obtained after oil extraction. Composition of oil cakes varies depending on their variety, extraction methods and growing condition. They have a high nutritional value and they used as animal feed because of their rich protein content (15 -50%). Oil cakes are good in fiber and non-starch polysaccharides (NSP). Their fat content depends on oil extraction method, which is usually less than 3-4% fat.1 Non-edible oil cakes such as Karanja cake, Castor cake, Neem cake (A. Indica) are used as nitrogenous fertilizer because of their high N, P, K content. Oilcakes protect the plants from insects, soil nematodes and parasite; there by offer great resistance to infection.2 A. Indica manure is by-product obtained after cold pressing of fruits and kernels and the solvent extraction process for A. Indica oil cake. Its organic manure contains organic nutrients like nitrogen, phosphorous, potassium, calcium, magnesium etc., including sulphur and bitter limonoids. A. indica cake organic manure is a proven soil conditioner and it is being used directly or in blends with other manures like, urea, Farmyard manure etc., for its benefits of organic nutrients, pest repellent properties and its control on soil bound nematodes.
Lipases are reported in various microorganisms and plants, apart from animal sources. Over the last decades interest in these enzymes has increased markedly, in view of their diverse applications in medicines, food additives, clinical reagents and cleaners and for synthesis of biodiesel and biopolymers. Microbial lipases have special industrial attention because of their selectivity, stability and broad substrate specificity.3,4 Microbial enzymes are highly stable in comparison of plant and animal enzymes and their production is also more safe and convenient.5 Therefore it is important to increase the production by optimizing culture conditions and at the same time reduce the production cost. A widely employed strategy for this is solid state or submerged fermentation modifications for microbial growth. However solid state fermentation (SSF) is most appropriate process due to its various benefits and bioconversion parameters.6,7 Crop residue as husk, fruit seeds, bran and bagasse are utilized as a potential organic material because they provide an excellent substratum for growth of organism supplying the required nutrients to them in bioprocesses.8,9 Most of the lipase research focuses on production and improvement of extracellular lipase through microorganisms. Technique of semi solid state fermentation (SmSSF) involves and growth of microorganisms on solids with free flowing water.
Previous studies done in our laboratory have identified a novel high lipolytic strain of P. aeruginosa JCM5962(T), isolated from soil of sugarcane field. The isolate produced an extracellular lipase of 31kDa with metal cobalt as an enhancer. 10 Present work was undertaken to study the potential of AIOC as semi solid state fermentation (SmSSF) medium for improved production of extracellular lipase from JCM5962(T).
Materials and Method
Bacterial Strain
P. aeruginosa JCM5962(T) was used throughout the study. The strain has been isolated from soil and identified by 16S rRNA sequencing (data communicated). It produced an extracellular lipase of 31kDa after 24 hour of incubation at 37ºC. The enzyme was characterized to be stable at 50ºC and at pH 8.0. The stock culture was maintained on 1:1 ratio of Nutrient broth (Hi-Media) and glycerol at −20°C. Working culture was prepared on nutrient broth for 24 h at 37°C.
Inoculum Preparation
A loopful of cells of lipolytic isolate P. aeruginosa was transferred to a 100 mL nutrient broth. It used as an inoculum after of incubation at 37°C for 24 h. Two hundred micro litres of inoculum (approximately 2×106 cells) was added to the oil cake media for fermentation.
Semi-Solid State Fermentation
The AIOC substrate used for the production of lipases, it was procured from local market. Dry A.Indica oil cake was taken with 100 ml of distilled water. The medium were mixed and autoclaved for 15 min at 121°C. The sterilized medium was used as SmSSF throughout the study.
Extraction of Enzyme
Phosphate buffer of pH 8 was added to the medium in 1: 1 ratio after fermentation and enzyme was extracted by centrifugation at 4°C at 10,000 rpm for 10 min. The clear supernatant obtained was used as crude enzyme and stored in sterilized vials for further use.
Lipase Assay
Lipase activity was studied using p-nitrophenyl palmitate (pNPP) as a substrate according to the protocol described previously10. Substrate solution containing phosphate buffer (pH 8.0) with gum Arabic (Hi Media) and sodium deoxycholate (Sigma Aldrich, USA) along with p nitrophenylpalmitate (Sigma Aldrich, USA) in isopropanol was pre-incubated with crude enzyme at 37°C. After incubation, the reaction was stopped by adding 0.5 mL of 3M HCl. The reaction mixture was centrifuged at 10000 rpm for 10 minutes at room temperature. The amount of p-nitrophenol released was spectrophotometrically measured at 405 nm.
Optimization of Production Parameters for SmSSF Using Oil Cakes
To obtain maximum lipase production to suit industrial processes, optimization of parameters and manipulation of composition of medium was done. Various parameters were optimized for maximal lipase yield which are incubation time (24, 48, 72 and 96 h) and the effect of carbon sources (1% w/v of glucose, fructose, sucrose, lactose, maltose), lipid materials as triglycerides (0.5% v/v) such as flax, mustard, sesame, soybean, and olive oil and nitrogen sources (1% w/v ammonium nitrate, potassium nitrate, peptone, beef extract, yeast extract) in the AIOC SmSSF were also examined.
Mixed Substrate Fermentation
The mixed media used in the study were designated A to F as given in table 1. The mixed substrate was prepared by mixing two different oil cakes in 1:1 ratio and preparing the SmSSF as described before. Best mixed preparation was selected; the above optimized ingredients were also tested for mixed substrate enrichment. The lipase activity was measured for each combination and presented as percent activity.
Table 1: (Mixed substrate used in the study).
| S.No. | Combinations of Oil Cakes | |
| 1 | Control | A. indica oil cake (AIOC) |
| 2 | Media A | A. indica oil cake (AIOC) + Flax oil cake (FOC) |
| 3 | Media B | A. indica oil cake (AIOC) + Mustard oil cake (MOC) |
| 4 | Media C | A. indica oil cake (AIOC) + Caster oil cake (CastOC) |
| 5 | Media D | A. indica oil cake (AIOC) + Coconut oil cake (COC) |
| 6 | Media E | A. indica oil cake (AIOC) + Groundnut oil cake (GOC) |
| 7 | Media F | A. indica oil cake (AIOC) + Sesame oil cake (SOC) |
Results and Discussion
An important aspect of prime importance in enzyme production is development of an optimum substrate. In the present study, the production of bacterial lipase by P. aeruginosa JCM5962(T) was studied in semi-solid state fermentation using AIOC as substrate.
Optimization of AIOC Concentration
Lipase activity in Sm-SSF was compared with different AIOC concentration (2%, 4%, 6%, 8% and 10%) at 37ºC. All media were inoculated with equal number (2×106 cells) of microorganisms and incubated at 37ºC. The lipase activity was measured in each and relative activity was calculated and presented. As shown in figure 1, the lipase activity was reported maximum at 4% concentration of IAOC which is about 6.16 U/gds. All further experiments were continued with same concentration of substrate (4%).
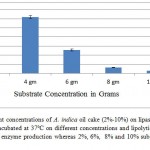 |
Figure 1: Effect of different concentrations of A. indica oil cake (2%-10%) on lipase activity by P. aeruginosa JCM5962(T ):
|
strain was incubated at 37ºC on different concentrations and lipolytic activity was recorded, 4% AIOC gave the maximum enzyme production whereas 2%, 6%, 8% and 10% substrate gave negligible lipase activity.
Optimization of Incubation Time
To determine incubation time for maximum lipase production, cultures were kept at 37ºC for 24, 48, 72, 96, 120 and 144 hours. As demonstrated in figure 2, in earlier stages of incubation a low level of lipase activity was obtained, which gradually increased with the passage of time and highest enzyme activity (17.236 U/gds) was obtained after four days (96 hours). The gradual decrease in the enzyme activity was observed with increasing incubation time beyond 96 hours which could be because of exhaustion of nutrients and production of toxic metabolites suggesting the enzyme being produced in the log phase of the growth.11,12 So the further experiments, the lipase production were studied after 96 hours culture of p. aeruginosa at 37ºC. Several reports showed the effect of incubation time on lipase production. Serratia marcesecens showed a good lipase activity after 6 days of incubation13 and Psychrobacter sp. Ant300,14 Pseudoalteromonas sp. wp2715 produced cold active lipase after 14 days of incubation.
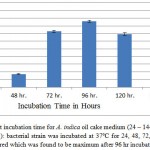 |
Figure 2: Effect of different incubation time for A. indica oil cake medium (24 – 144 hours) on lipase activity by P. aeruginosa JCM5962(T ): bacterial strain was incubated at 37ºC for 24, 48, 72, 96, 120 and 144 hours and lipolytic activity was measured which was found to be maximum after 96 hr incubation (17.24 U/gds).
|
Effect of Carbon Sources
The impact of additional 1% (w/v) carbon sources at was studied (figure 3). Among the tested carbon sources, it was found that, lactose induced maximum production of lipase (90%) which was 10% less then control (AIOC without any additional nutrient). However in this study, the enhancing effect in lipase production by supplying different carbon sources was limited, the maximum lipase producing carbon source was found to be lactose which also did not increase enzyme production. Whereas the results reports of Alkan et al.16 stated that the lipase production by Bacillus coagulants under SSF using melon wastes was highly influenced by sucrose. And also maximum lipase production by Pseudomonas aeruginosa PseA in sucrose supplied medium.17
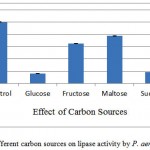 |
Figure 3: Effect of different carbon sources on lipase activity by P. aeruginosa JCM5962(T ):
|
Glucose, fructose, maltose, sucrose and lactose were used as additional nutrient ion, lactose was found to give 90.56% activity in comparison to control. It shows no additional carbon sources required in IAOC medium.
Effect of Nitrogen sources
Nitrogen source is another important factor in any fermentation process for successful production of biomolecules. The effect of organic and inorganic nitrogen sources in SmSSF is shown in figure 4. Ammonium nitrate, potassium nitrate, peptone, beef extract and yeast extract (1% w/v) when used as additional sources they increased the production of lipase from P. aeruginosa upto 179.47%. Ammonium nitrate was found the best additional source as it increased 79% lipase activity in comparison of AIOC without any additive. However, supplementation of peptone was found to have no significant role in increasing the production. An increase in lipase production when ammonium nitrate was added as inorganic nitrogen source to the Geotrichum candidum has been shown.18 According to one report that lipase production by Rhizophus homothallicus under SSF was highly influenced by ammonium nitrate supplied medium.19
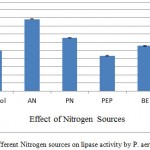 |
Figure 4: Effect of different Nitrogen sources on lipase activity by P. aeruginosa JCM5962(T ):
|
Supplemenatry nitrogen sources were used as for the AIOC medium, in which AN was found to give 176.49% activity in comparison to control. AN – Ammonium nitrate, PN – Potassium nitrate, PEP – Peptone, BE – Beef extract, YE – Yeast extract.
Effect of Lipid sources
The supplementation of different triglycerides, i.e. flax, sesame, mustard, olive, and soybean oil at concentration of 0.5% were added to the AIOC SmSSF medium and lipase activity was detected. The results in the present study revealed that (Figure 5) only mustard oil increased lipase activity marginally (4.12%) increase) when compared with control, whereas rest of the oil supplements suppressed the lipase production. The reason for this may be presence of required lipids in oil cake itself. According to Eitenmiller et al.20 it may also be due to the fact that higher oil content resulted in the formation of a biphasic system. In the study inhibited production of lipase by Penicillium roqueforti was seen by butter oil, corn oil, or olive oil. Whereas Lima and coworkers21 reported that olive oil induced higher yield of lipase by Penicillium aurantiogriseum.
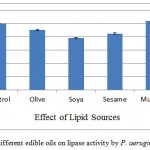 |
Figure 5: Effect of different edible oils on lipase activity by P. aeruginosa JCM5962(T ):
|
Olive oil, soybean oil, sesame oil, mustard oil and flex oil (.5%) were used as additional triglycerides sources for the AIOC medium. Mustard oil was found to give 104.12% activity in comparison to control whereas all others inhibited the lipase activity. It shows AIOC did not need any additional lipid source.
Effect of Mixed Substrate
Further studies were made to evaluate the additive effect of other oil cakes by mixing them with the AIOC in the ratio of 1:1. Preparations of various mixed substrates of AIOC along with sesame, flax, caster, mustard, groundnut and coconut oil cake were made. As represented in figure 6, coconut oil cake was found to be the most suited for mixed substrate with 38% increase in lipase production after 96 hours of incubation at 37°C followed by mustard oil cake (25.95%) and flax oil cake (5.6%). However castor, groundnut and sesame oil cakes inhibited the enzyme production. Each combination was also tested with 1% w/v nitrogen source (ammonium nitrate) and though an increase in production was obtained, it was not so significant with highest yielding mixed substrate (AIOC+COC). Another interesting observation was 29% inhibition in enzyme activity with AIOC and GOC mixed substrate whereas 172% up regulation in lipase production in the same SmSSF medium (AIOC+GOC) supplemented with NH4NO3. In other similar work, increased production of bacterial lipase was found with combination of glucose and ammonium nitrate.22 Similarly, a very good lipase production by Aspergillus niger by optimizing mixed substrate in ratio of 1: 1 was obtained.23
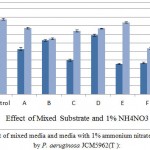 |
Figure 6: Effect of mixed media and media with 1% ammonium nitrate on lipase activity by P. aeruginosa JCM5962(T ):
|
Mixed media, A (AIOC with FOC), B (AIOC with MOC), C (AIOC with castOC), D (AIOC with COC), E (AIOC with GOC) and F (AIOC with SOC) along with control (AIOC) were studied and each combination was also tested with 1% ammonium nitrate as additional nitrogen source. Results presented as percent activity ± SD, revealed AIOC combined with coconut oil cake in 1:1 ratio gave maximum activity which is 38% more than control (AIOC).
This study correlates that lipase production by AIOC and groundnut oil cake under SSF was highly influenced by ammonium nitrate supplied medium. Similarly many reports indicate that the lipase production was high in ammonium nitrate added medium.21
Conclusion
One of the key areas in modern industrial biotechnology is optimum use of by-products of agriculture waste for growth of microorganisms or production of enzymes (e.g. residues from oil seeds). According to present work, the extracellular lipases could be economically produced by a novel high lipolytic P. aeruginosa JCM5962(T ) by semi-solid state fermentation using easily available, non edible and low cost A. Indica (neem) oil cake as potent substrate. However, blending of A. Indica oil cake along with coconut, mustard and flax oilcake under optimized condition showed better yield of lipase. AIOC along with COC and ammonium nitrate has been found to be a potential substrate for industrial production of extracellular lipase in SmSSF. It is also reported that production of lipase through low cost A. indica oil cakes SmSSF might not only reduce the cost of production but also result in many fold increase in the lipase yield when compared with submerged fermentation.
Acknowledgement
The authors extend gratitude to Amity University Lucknow Campus for extending necessary facilities to carry out this work.
Conflict Of Interest
The authors [Shweta Sachan and Aditi Singh (corresponding author)] are declaring that there is no conflict of interest regarding the publication of this paper.
References
- Li N., Zong M. H. Lipases from the genus Penicillium: production purification characterization and application. Mol. Catal. B: Enzym. 2010;66:43-54.
CrossRef - UK Essays. Use of solid state fermentation web. Available from. 2013. https://www.ukessays.com/essays/engineering/use-of-solidstate-fermentation-in-the.php?cref=1
- Dutra J. C. V., Terzi S. C., Bevilaqua J. V., Damaso M. C. T., Couri S. Langone M. A. P. Lipase production in solid state fermentation monitoring biomass growth of Aspergillus niger using digital image processing. Biochem. Biotechnol. 2008;147:63-75.
CrossRef - Griebele N., Polloni A. E., Remonatto D., Arbter F., Vardanega R., Cechet J. L. et al., Isolation and screening of lipase producing fungi with hydrolytic activity. Bioprocess. Tech. 2011;4:578-586.
CrossRef - Hasan F., Shah A. A., Hameed A. Industrial applications of microbial lipases. Microb. Technol. 2006;39:235-251.
CrossRef - Zhang L. Q., Zhang Y. D., Xu L., Yang X. L., Yang X. C., Xu X. L., Wu X. X., Gao H. Y., Du W. B., Zhang X. Z. Lipase catalyzed synthesis of RGD diamide in aqueous water-miscible organic solvents. Microb. Technol. 2001;29:129-135.
CrossRef - Sharma R., Chisti Y., Banerjee U. C. Production, purification, characterization and application of lipases. 2001;19: 627-662.
CrossRef - Pandey A., Soccol C. R. Economic utilization of crop residues for value addition- a futuristic approach. Sci. Ind. Res. 2000;59:12-22.
- Pandey A., Soccol C. R., Mitchell D. New developments in solid state fermentation, I: Bioprocesses and product. Biochem. 2000;35:1153-1169.
CrossRef - Sachan S., Chandra V. Y., Yadu A., Singh A. Cobalt has enhancing effect on extracellular lipases isolated from Pseudomonas aeruginosa JCM 5962(T). J. Pharm. Tech. Res. 2017;10(1):45-49.
- Alagarsamy S., Paul D., Chandran S., Szakacs G., Soccol C. R. Rice bran as a substrate for proteolytic enzyme production. Arch. Biol. Technol. 2006;49:843-851.
CrossRef - Haq I. U., Mukhtar H., Umber H. Production of protease by Penicillium chrysogenum through optimization of environmental conditions. Agri. Soc. Sci. 2006;2(1):23-25.
- Lee H. K., Ahn M. J., Kwak S. H., Song W. H., Jeong B. C. Purification and characterization of cold active lipase from Psychrotrophic aeromonas LPB 4. J. Microbiol. 2003;41(1):22–27.
- Kulakova L., Galkin A., Nakayama T., Nishino T., Esaki N. Cold-active esterase from Psychrobacter Ant300: gene cloning, characterization, and the effects of Gly→Pro substitution near the active site on its catalytic activity and stability. Biochimica. et. Biophysica. Acta. 2004;1696(1):59–65.
CrossRef - Zeng X., Xiao X., Wang P., Wang F. Screening and characterization of psychotropic, lipolytic bacteria from deep-sea sediments. Microbiol. Biotechnol. 2004;14(5):952–958.
- Alkan H., Baysal Z., Uyar F., Dogru M. Production of lipase by a newly isolated Bacillus coagulans under Solid-State Fermentation using melon wastes. Biochem. Biotechnol. 2007;136:183-192.
CrossRef - Mahanta N., Gupta A., Khare S. K. Production of protease and lipase by solvent tolerant Pseudomonas aeruginosa Pse A in solid-state fermentation using Jatropha curcas seed cake as substrate. Technol. 2008;99:1729-1735.
- Gopinath S. C. B., Hilda A., Priya T. L., Annadurai G., Anbu P. Purification of lipase from Geotrichum candidum: conditions optimized for enzyme production using Box-Behnken design. J. Microbiol. Biotechnol. 2003;19(7):681–689.
CrossRef - Rodriguez J. A., Mateos J. C., Nungaray J., Gonzalez V., Bhagnagar T. Improving lipase production by nutrient source modification using Rhizopus homothallicus cultured in solid state fermentation. Biochem. 2006;41:2264-2269.
CrossRef - Eitenmiller R. R., Vakil J. R., Shahani K. M. Enzymes of food industry. Sci. 1970;35:130–133.
- Lima V. M. G., Krieger N., Sarquis M. I. M., Mitchell D. A., Ramos L. P., Fontana J. D. Effect of nitrogen and carbon sources on lipase production by Penicillium aurantiogriseum. Technol. Biotech. 2003;41:105-110.
- Joseph B., Upadhyaya S., Ramteke P. Production of cold-active bacterial lipases through semisolid state fermentation using oil cakes. Res. 1011;2011:1-6.
CrossRef - Pau H. S., Omar I. C. Selection and optimization of lipase production from Aspergillus flavus USM A10 via solid state fermentation on rice husks and wood dusts as substrates. Pak. J. Biol. Sci. 2004;7:1249–1256.
CrossRef`

This work is licensed under a Creative Commons Attribution 4.0 International License.





