How to Cite | Publication History | PlumX Article Matrix
Synthesis of Magnetite Nanoparticles by Biological Technique
Hamid Reza Ghorbani, Hossein Pazoki and Ali Shokuhi Rad
Department of Chemical Engineering, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran.
Corresponding Author E-mail: hamidghorbani6@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2488
ABSTRACT: The development of synthesis routes for oxide nanoparticles is a matter of considerable topical attention. Green synthesis of nanoparticles with the help of microorganisms as reducing agents is an efficient, cost effective, fast and eco-friendly in nature. This paper presents a simple technique to synthesize magnetite (Fe3O4) nanoparticles. In this routine, an aqueous solution of ferrous and ferric salts was mixed with Magnetospirillum and heated for 10 minutes at 70℃. UV–vis absorption spectra, dynamic light scattering (DLS) and transmission electron microscopy (TEM) have been used to illustrate the form process and explain the structure of the magnetite nanoparticles. UV–Vis absorption spectrum showed surface plasmon resonance absorption bands about 240 nm that confirmed magnetite nanoparticles existence. We obtain magnetite nanoparticles of size 42±20 nm after separation and washing procedures by dynamic light scattering (DLS).
KEYWORDS: Characterization Magnetospirillum; Magnetite nanoparticles;
Download this article as:| Copy the following to cite this article: Ghorbani H. R, Pazoki H, Rad A. S.Synthesis of Magnetite Nanoparticles by Biological Technique. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Ghorbani H. R, Pazoki H, Rad A. S.Synthesis of Magnetite Nanoparticles by Biological Technique. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=24662 |
Introduction
Green synthesis of nanoparticles is a perfect field of nanotechnology, which attracts great attention in the fields of medicine, pharmaceutical, electrical, technological and other science research areas. Mostly the nanoparticles synthesized through different chemical and physical methods, these approaches are complicated, expensive and cause potential environmental and biological hazards. Green synthesis of nanoparticles with the help of microorganisms as reducing agents is an efficient, cost effective, fast and eco-friendly in nature. In recent past, most of the scientist adopted green synthesis methods for the production of a narrow range of different types of nanoparticles like, copper, gold, iron, silicon, silver and zinc from various microorganisms.1,2
Different techniques of synthesizing magnetite nanoparticles have been developed such as sol gel synthesis, chemical vapor condensation, flame synthesis, laser ablation and reverse microemulsion.3 These chemical and physical methods owe some disadvantages like, sol gel synthesis has high raw materials cost, shrinkage and cracking may occur during the drying process and need very high temperature furnace or heating device. The chemical vapor condensation method requires higher temperature usually between 300-900°C, has a slower growth rate and usage of precursors are highly toxic (Ni(CO)4), explosive (B2H6) or corrosive (SiCl4). Laser ablation method limits wavelength of the laser impinging the metallic target, the duration of the laser pulses, the laser fluence, the ablation time duration and the effective liquid medium, with or without the presence of surfactants. Reverse microemulsion method limits its high cost and difficulties in removal of surfactants in.4,5,6 In this study, we suggest a rapid, non-toxic, simple and biological synthesis method to form magnetite nanoparticles in only one step.
Materials and Methods
Ferrous chloride tetra-hydrate, ferric chloride hexa hydrate, and sodium hydroxide (NaOH) were obtained from Merck Co. as analytical grade. Magnetite nanoparticles (Fe3O4) synthesized as follows: 0.45 g of FeCl2.4H2O and 0.96 g of FeCl3.6H2O were dissolved in 100 mL of sterile deionized water in a 250 mL beaker and heated at 70℃ under mild stirring. After 15 minutes, 4 mL of the aqueous solution of Magnetospirillum was added to this solution, instantly the colour of the mixture changed from yellow to reddish brown. After 15 minutes, 10 mL of sodium hydroxide aqueous solution was added to the mixture with rate 5 mL/min to precipitate magnetite nanoparticles. After addition of sodium hydroxide, the reddish-brown solution changed to black suspension. The solution was cooled to room temperature and the magnetite nanoparticles were achieved by centrifugation. The magnetite nanoparticles were washed by dispersing in distilled water and centrifugation two times. The magnetite nanoparticles after washing were dried 12 hour at 80℃ for Uv-vis spectroscopy, TEM and DLS analysis.
Results and Discussions
The peak at about 240 nm is known to be due to surface plasmon resonance in magnetite nanoparticles solutions. The UV–Visible spectrum of the solution displayed a clear surface plasmon resonance at ∼240 nm (Fig. 1). The method mentioned has confirmed to be very suitable for analyzing nanoparticles.7
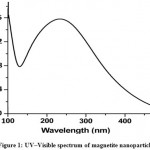 |
Figure 1: UV–Visible spectrum of magnetite nanoparticles
|
Dynamic light scattering is an appropriate procedure for the measurement of nanoparticles size. The magnetite nanoparticles’ size histograms display that the nanoparticles size is 42± 20 nm (Fig. 2). Typical electron micrograph of nanoparticles on surface obtained by TEM technique for prepared samples and is shown in Fig.3.TEM micrograph of magnetite nanoparticles is shown in Fig. 3.
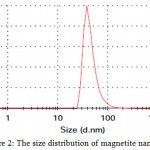 |
Figure 2: The size distribution of magnetite nanoparticles
|
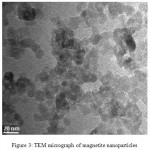 |
Figure 3: TEM micrograph of magnetite nanoparticles
|
Magnetite synthesis by Magnetospirillum bacteria and Fe(III)-reducing bacteria has been widely studied. In contrast, whether magnetite can be produced by Fe(II)-oxidizing bacteria stayed still obscure. In this work, we experimentally indicate that stable magnetite nanoparticles is formed using the nitrate reducing Fe(II)-oxidizing. Also the possibility of oxide nanoparticles synthesis using bacteria has been studied. Most of the works in this matter is centralized on production of magnetite nanoparticles, by magnetotactic bacteria (in nature). In these researches, nanoparticles synthesis was very slow under anaerobic conditions. It was exciting to understand the capability of bacterium Actinobacter sp to produce magnetite and magnetite under fully aerobic conditions. In this work, we showed the synthesis of magnetite nanoparticles using Magnetospirillum.
Conclusions
Magnetite nanoparticles were produced by a biological, rapid and simple technique. UV–Vis spectroscopy confirmed magnetite nanoparticles. Also, the average diameter of magnetite nanoparticles was measured about 42 nm using DLS analysis. In addition, nanoparticles were observed by TEM and results were confirmed. In current study, the biological technique has beneficial features for production magnetite nanoparticles due to environmentally friendly, non-toxic and economical.
References
- Wang B. S., Chia P. J.,Chua L., Zhao L. H., Png Q. R., Sivaramakrishnan S., Wee S., Ho H. Band-like Transport in Surface-Functionalized Highly Solution-Processable Graphene Nanosheets. Adv. Mater. 2008;20:3440–3446.
CrossRef - Karousis N., Sandanayaka A. S. D., Hasobe T., Economopoulos S. P., Sarantopouloua E., Tagmatarchis N. Graphene oxide with covalently linked porphyrin antennae: Synthesis, characterization and photophysical properties. J. Mater. Chem. 2011;21:109-117.
CrossRef - Hamed M. A. Pre-concentration and separation of Fe (III), Co (II), Ni (II) and Zn (II) by solid-phase extraction using silica modified with N-propylsalicylaldimine. Egypt. J. Aquat. Res. 2005;31(1):31-41.
- Huang X., Chang X., He Q., Cui Y., Zhai Y., Jiang N. Tris(2-aminoethyl) amine functionalized silica gel for solid-phase extraction and preconcentration of Cr(III), Cd(II) and Pb(II) from waters. J. Hazard. Mater. 2008;157:154-160.
CrossRef - Ghaedi M., Fathi M. R., Shokrollahi A., Shajarat F. Highly selective and sensitive preconcentration of mercury ion and determination by cold vapor atomic absorption spectroscopy. Anal. Lett. 2006;39:1171-1185.
CrossRef - Mamania J. B., Costa-Filhob J., Cornejoc D. R., Vieirad E. D., Gamarraa L. F. Synthesis and characterization of magnetite nano particles coated with lauric acid. Mater. Charact. 2013;81:28-36.
CrossRef - Meyer J. C., Geim A. K., Katsnelson M. I., Novoselov K. S., Booth T. J., Roth S. Nature. 2007;446:60-68.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





