How to Cite | Publication History | PlumX Article Matrix
Saba Aslani1,2, Ghasemali Garoosi1 and Hossein Jafary3
1Faculty of Agriculture and Natural Sciences, Imam Khomeini International University, Qazvin, Iran.
2Department of Biotechnology,Imam Khomeini International University, Qazvin, Iran.
3Jehad Agriculture Organization of Zanjan Province, Zanjan, I.R. Iran.
Corresponding Author E-mail: saba.aslani@chmail.ir
DOI : http://dx.doi.org/10.13005/bbra/2501
ABSTRACT: Verticillium wilt, which is caused by the fungus Verticillium dahliae, is one of the most important olive diseases worldwide. There are many ways to extract DNA from plant pathogenic fungi and from plant tissues for molecular-based diagnostic assays. LAMP is a new and sensitive molecular-based technique used for detection of plant pathogenic agents with minimum requirements needed. In this study, we tried to achieve a simple, cost effective and efficient method of DNA extraction from both Verticillium dahliae fungus and from infected wood samples in order to run a loop-mediated isothermal amplification (LAMP) assay. Efficiency of three DNA isolation methods from both mycelia and infected wood samples was evaluated. For this purpose, wood samples from infected olive trees were collected from Tarom region in Zanjan province and the samples were cultured on the media. The fungus was isolated and identified as V. dahliae based on morphological features. Then the genomic DNA was extracted using traditional CTAB method, fast NaOH method and direct isolation method from infected wood samples. After assessment of the quality and the quantity of the extracted DNA samples, a LAMP assay was ran using specific primer pairs and the DNA templates extracted using three different methods. In spite of the significant differences in the quantity of DNA samples, LAMP assay could successfully detect the fungus in all samples. The improved direct isolation of the DNA of V. dahlia from infected wood, followed by a LAMP assay could considerably shortened the detection process of the fungus and hence is a suitable method for screening of olive trees and saplings against Verticillium wilt disease.
KEYWORDS: DNA isolation Molecular detection; Olive; LAMP; Verticillium dahliae;
Download this article as:| Copy the following to cite this article: Aslani S, Garoosi G, Jafary H. Using a Loop-Mediated Isothermal Amplification (LAMP) Assay and Direct DNA Extraction Method from Wood for Rapid Detection of Verticillium dahliae in Olive Trees. Biosci Biotech Res Asia 2017;14(2). |
| Copy the following to cite this URL: Aslani S, Garoosi G, Jafary H. Using a Loop-Mediated Isothermal Amplification (LAMP) Assay and Direct DNA Extraction Method from Wood for Rapid Detection of Verticillium dahliae in Olive Trees. Biosci Biotech Res Asia 2017;14(2). Available from: https://www.biotech-asia.org/?p=25423 |
Introduction
Verticillium wilt, is one of the important and damage-causing olive diseases worldwide (Rodriguez-Jurado, et al., 1993). At present time, this is considered as the most important olive disease in Golestan Province, Iran (Attar et al., 2011). In recent years, Verticillium wilt has been improved due to extension of olive planting in infected soils and distribution of infected scions. Accordingly, multiple cases of infected tress have been observed in Zanjan and Golestan Provinces, Iran. In addition, the disease has been spread in other olive producing areas of Iran, as infection index of gardens in the country was estimated to be 12% (Afshari Azad et al., 2001, Attar et al, 2011). In Zanjan Province, olive Verticillium wilt is considered as the second main fungus disease of olive, placed after root rot disease caused by the Armillaria mellea (Jafari et al., 2011). Detection of Verticillium wilt via its symptoms is not an easy task, because a vast range of biotic and abiotic stress factors may cause similar symptoms in love. In order to diagnose the infection of olive trees by Verticillium dahlia, the fungus may be isolated using conventional cultivation methods in the general or special media.
Due to poor saprophytic power of fungi and its slow growing rate, this technique seems to be time-consuming and inefficient for detection of hidden infections. In recent years, some PCR-based markers have been presented for specific detection ofpathogenic V.dahliae isolates, which could also be used for tracing of fungus Verticillium in olive (Usami,et al., 2000). Application of PCR-based molecular technique, despite its numerous and significant advantages, has its own weaknesses, including high cost of laboratory equipment such as a thermo cycler and Duct gel.
During the last 10 years, due to simplicity, high rate and high efficiency, LAMP (loop-mediated isothermal amplification) technique has been frequently used for diagnosis of microorganisms. LAMP technique has been firstly introduced by Notomi et al., (2000). In this method, a polymerase DNA and a set of four specifically designed primers are used to detect six specified area of the target DNA. No expensive equipment namely thermo cycler is required and the steps are more feasible than other techniques such as PCR and real-time PCR (Fu et al, 2010). Simple implementation is the major advantage of this method, which requires only primers, Bst polymerase enzymes, hot water bath or a simple heating block for reactions in a laboratory. Another important aspect of this methodis the ability todetect the positive reaction via turbidity, which is caused by magnesium pyrophosphate precipitate formed during the reaction. This way, practically it is not required to load the reaction product on electrophoresis gel. There are numerous reports proving that the method is a suitable molecular tool to amplify the target nucleic acid successions (Fang et al., 2008). A large content of DNA product is amplified during LAMP reaction (more than 45microgram of target DNA per 50 microliter of reaction mixture), (Nagamine et al., 2002).
In the past, LAMP product became visible by agarose gel electrophoresis and detection by ethidium bromide, but by now it is possible to detect the final products of reaction by clouding of reaction media.Lack of need to denaturation of initial model for initiation of polymerization reaction and even extraction of initial model from some biological samples (liquid cultivation media), are some of the advantages of this method, which are unique among the amplification method of nucleic acids (Chen et al., 2008, Imai et al., 2006).No need to special equipment used for PCR (such as the costly thermo cycler device) to control sudden temperature changes (Imai et al., 2006, Mori et al., 2006) and high sensitivity to amplify the target DNA sequence, high specificity, simple amplification, feasibility, high efficiency, cost-effectiveness in terms of cost and time could be mentioned as the advantages of this technique (Fang et al., 2008).
LAMP technique has been used extensively in the diagnosis of DNA or RNA-viruses. Fukuta et al., (2003), have used LAMP technique for diagnosis of Tomato yellow leaf curl virus (TYLCV). In recent years, LAMP technique has been successfully used for diagnosis of Mycobacterium bovis (Zhang et al., 2010) and Vibrio cholera, (Okada et al., 2010) Pathogenic bacteria.
Up to now, only few cases have been reported about application of LAMP technique for identifying the fungi and semi- fungi organisms including the diagnosis of Phytophthora ramorum oomycetes (Tomlinson et al., 2007), Glomus intraracices arbuscular mycorrhizal fungi in carrots (Gadkar and Rillig , 2008) and Fusarium graminearum pathogenic fungus in cereals (Niessen and Vogel, 2010).
In Iran,the technique has been used to detectSalmonella (Moradi et al. 2009) Potato leaf curl virus (Diamond et al. 1390) and cucumber mosaic virus (Ghasemi et al. 2012) so far. Recently some researches have been conducted by Jafari et al., (The article is under printing) to detect the Verticillium fungus. Inmentioned research, the CTAB method was used to isolate the DNA of fungal mycelium and it was concluded that LAMP technique is more sensitive than other molecular assay methods such as Nested-PCR for detection of Verticillium fungus. However, cultivation of infected plant samples, isolation and purification of the fungus and DNA extraction steps are highly time-consuming.
In this paper, in addition to the conventional CTAB methods, an alternative method is also used for extraction of DNA from woody tissue. Besides, LAMP reaction was directly implemented using infected wood samples (without extraction of DNA). Regarding the high sensitivity of LAMP technique, direct isolation of fungi from infected woods makes it possible to diagnose and detect the infection in mother trees immediately. This may be so helpful in production of healthy trees free of infections and diseases, and control the spread via plant proliferation materials.
Materials and Methods
Sampling of olive groves
Sampling of infected twigs and stems showing symptoms such as wilting, drying, falling leaves and browning of vascular green, was performed on olive groves in Tarom city, Zanjan, Iran over the years.
Some fragments with length of about 3 inches were provided from the suspected stem, which were thansurface sterilized for 2 minutes usingcommercial sodium hypochlorite and distilled water (1: 9) solution. In continue using the sterilized scissors the top and bottom of samples were cut off and the remaining skin-free partswere divided insmaller triangular pieces. They were then transferred to PDA cultivation media and restored for 12 days in darkness at 24°C.
After colonization of the fungus around the segments of cultured stem, purification steps were implemented using single-spore cultivation method.
Extraction of DNA from the Verticillium mycelium fungus using the CTAB Method
A piece of 0.5cm dimension was isolated from the pure colonies of fungi, which were purified in the PDA solid cultivation media. Under sterile conditions it was transferred to a 500 ml Erlenmeyer flask containing 150ml of PDB liquid medium. The flasks were put under stirring rate of 88rpm for three days at 24ᵒC to accelerate the growth of fungi and provide the liquid medium with adequate oxygen content.Since then, flasks were restored in darkness (24ᵒC) for the duration of 12-14 days to form a layer of mycelium on the upper surface of the PDB media.
In order to separate the mycelium fungi from the liquid medium, the content of the fungi-containing flask was passed through the sterilized tiffany and the samples were centrifuged at 3000 rpm for 2 minutes. Then, slowly,sterilized tiffanies were removed from the falcons on the ice pieces, as they did not contact with water. Dewatered mycelium fungi was poured in a mortar and turned into a soft powder using liquid nitrogen.
Fast Extraction of DNA fom Mycelium
In this step, NaOH method was used for extraction of DNA (Wang et al.). To do this, first the extraction solutions have been prepared including NaOH 0.5MTris-HCL, 100mM, PH=8.20μl of 0.5M NaOH were added to 2ml tubes containing 2mg of powdered mycelium fungi in liquid nitrogen, followed by 5 minutes of stirring. Then, adequately, 20μl of Tris-HCL 100mM were added and the tubes were gently upside down. In the next step, 5μl of supernatant was removed and transferred to another sterilized tube, containing 200μl of Tris-HCL 100Mm, pH=8. It was then stored at -20ᵒC for future applications.
Direct Extraction of DNA from Woody Tissue
Some pieces with the length of about 20-30 cm were prepared from olive wood, as their infection by fungus Verticillium have been previously approved via conventional cultivation methods (culture, purification and single spores) was approved.Some pieceof the length of about 20-30cm have been prepared from olive wood, as their negative infection to fungus Verticillium was previously approved via conventional techniques (cultivation, purification and single spore). Three samples of healthy woods (supplied form non-infected trees) have been prepared. Then, modified CTAB technique was used to extract the DNA from the infected tissue of wood. Wood particles were also prepared via sawing. To do this, first theupper layer of surface was removed and the first layer between the bark and wood was sawed and turned into powder.
In this method, there is no need to prepare powders in liquid nitrogen. Extraction buffer containing1/4mM NaCl, 20mM EDTA, 100nM Tris-Hcl, 1% PVP and 2% CTABwas warmed up in hot water bath for 10 to 15 minutes at 65°C. Then, 1000μl of buffer was extracted and added to each of 2ml tubes containing 0.2g wood powder. This was continued by adding 2μl of 2-mercapthanol solution to each tube. The tubes were then placed in hot water bath (65°C)and gently upside down every 15 minutes. After removing the samples from hot water bath, 500ml of chloroform isoamyl alcohol (24:1) was added to them and samples were vertexed for 8-10s. Afterwards, samples were centrifuged for the duration of 15 minutes at 12000rpm leading to distinction three special phases in each tube. The upper phase which contains DNA was transferred to another sterilized tube with caution. Then, the last step was performed again with some changes (centrifuge at 14000rpm). Isopropanol solution was added to the tube in the same volume of supernatant removed liquid. The tubes were then gently stirred and kept at -20ᵒC for 20 minutes. The samples were then put under centrifugation (9000rpm) for 8 minutes. The supernatant solution was removed with caution and DNA precipitates were prepared to be washed with 70% ethanol solution. After two washes, the alcoholic solution was discarded and the DNA-containing tube was let to be dried in the air for 15-20 minutes. The resulting dry precipitate was dissolved in 100µl TE buffer solution and stored at -20ᵒC for future applications.
Taking LAMP Reaction for Detecting the Fungi
Lampe reactions were performed using three types of extracted DNAs. In order to design the primers required for lamp reaction, a piece of DNA pertaining to V. dahliae fungi was used. It was previously sequenced to detect the pathotypes of this fungus (Mercado-Blancoet al, 2001). Design of primers has been performed by means of PrimerExplorer V software (the special online software for designing LAMP primers) (Jafari et al., under printing). Succession of the primers used in LAMP test has been presented in Table 1. LAMP reaction has been performed at final volume of 25µl (2 µl DNA, 1µl of 10dNTPmM, 3µl of 20FIPpmol primer, 3µlof BIPpmol primer, 2µl of 5F3pmol primer, 2µl of 5B3pmol primer, 2µl of LF5pmol primer, 2µl of LB5pmol primer, 2µl of 5M Betaine, 1µl of Bst DNA 8U polymerase enzyme, 1.5µl of 8mM MgSO4, 2.5µl of 10BstX buffer and 1µl sterilized water) at 60ᵒC for 60 minutes in a heating block. Upon completion of the reaction tubes were removed and the cloudy state of the reaction was positive. Then, 5µl of LAMP product was electrophoresized in 1%agarose gel using a 100DNA Ladderbp ladder marker. Photography was also performed after staining by ethidium bromide usinggel doc apparatus.
Table 1: the name and the sequences of primers used in the LAMP assay
| Sequence | Primers |
| 5′AACAGCGGAAGCCGTGTCAAAGTTTTAGCAAAGATACCGTGTCTGG3′ | FIP |
| 5 ′ TGCCAAGGCCATGTTTGCGTTTTTGCGAACACCAAACCCCTT 3′ | BIP |
| 5 ′ TCGAGAGCGCCTTCTTCT 3′ | F3 |
| 5 ′ GCCCGTCCATCTTCACCT 3′ | B3 |
| 5′ CTCACTCCTCCTGCCTCTCA 3′ | LF |
| 5 ′ TGCAGAGTGCGGATAGGAAA 3′ | LB |
Results and discussions
Isolation and Detection of Fungi V. Dahlaie
After 12 days, the colony isolates in PDA media were white to cream. In continue, due to formation of colonies of fungi sclerotia, fungus colonies were turned to black. Fungus colorless conidiophores have been formed vastly in the cultivation media. They raised with a semi-vertical direction. Each conidiophore possessed three Phialidesin the size of 16-35×1-2.5 micrometer, derived from the same point. Fungus Conidia were in the size of 4.2-7 × 2.8-4.9 micrometers formed as single or in small groups at the end of conidiophores.
Ellipticalto almost irregular cylindrical Conidia were transparent and single rooms. Thick and dark Mycelium have been formed in the culture exclusively with fine sclerotia. Sclerotia were formed in various sizes and shapes with a diameter of about 15-50 micrometer. Detection of fungus Verticillium species was donebased on morphological and morphometric Phialidesin,Conidia and Microsclerotia. Morphological studies approved that the isolates belonged to the V. dahliaesp species. Such characteristics were exactly in accordance with the fungus isolates sent to Iranian Research Institute of Plant who and its approved it was belonged to V. dahliae species.
Comparing three Methods of Fungus DNA Extraction
Efficiency of three methods of DNA extraction has been studied in this research. Four types of Verticillium isolates have been used for extraction of fungus mycelium via CTAB and NaOH methods. These isolates were separated and isolated from infected olive wood via ordinary extraction methods. In direct extraction from wood, three samples with isolated fungus were used, where two samples were put under DNA extraction and the third sample could not be treated in the same way. Evaluation of quantity of DNA extracted from isolates by means of a spectrophotometer and reading the absorbance at 260 and 280nm wavelength revealed that DNA value of fungus isolates in three methods is greatly different. In conventional extraction technique (CTAB), the average value of DNA concentration measured in all four isolates was about 4000nanogram/microliter, while for NAOH method the noted value was about 85nanogram/microliter, which is considerably lower than the previous one (Table 2).
Table 2: Concentration of DNA extracted using three different methods
| Method of DNA Extraction | Sample ID | Nucleic acid Conc. | Unit |
| (DNA Extraction from mycelia by using CTAB method) | DNA(1) | 862 | ng/µl |
| DNA(2) | 725 | ng/µl | |
| DNA(3) | 750 | ng/µl | |
| DNA(4) | 940 | ng/µl | |
| (DNA Extraction from mycelia by using NaOH method) | DNA(1) | 72 | ng/µl |
| DNA(2) | 62 | ng/µl | |
| DNA(3) | 87 | ng/µl | |
| DNA(4) | 121 | ng/µl | |
| (DNA Extraction from wood) | DNA(1) | 6.2 | ng/µl |
| DNA(2) | 8 | ng/µl |
The other problem was about instability of extracted DNA. Due to incomplete removal of NaOH and residual content in final DNA solution, it is not stable and approximately one week after extraction, the extracted DNA could not be reused for LAMP reaction. However, the major advantage of NaOH method is fast extraction of DNA. In comparison with CTAB method, relatively lower time is required for extraction, as DNA was prepared for LAMP reaction in less than 10 minutes. In direct extraction from wood, the content of extracted DNA was extremely lower than that of NaOH method. Also it was not possible to exactly determine that the ratio of extracted DNAbelonged to fungi or the plant.
It is expected that fungus DNA is only a small part of the total extracted DNA. Defect of this extraction method is about very low concentration of DNA and its combination with DNA of the host plan. But its main advantage is about direct and fast extraction of DNA from fungi, as in this method we do not need to cultivation, purification and isolation of fungus from infected tissue. Implementation of above procedures for olive needs to more than 20 days. In this study, some changes were made in CTAB method in order to extract DNA from infected and healthy wood tissues. In conventional methods used for extraction of DNA from wood, wooden fragments are cut to finer pieces using special mil and liquid nitrogen. In the method used here, the particles are directly obtained from sawing the tissue between bark and wood. The resulting sawdust is soft enough and there is no need to grind their again in liquid nitrogen. In addition to feasibility, this technique reduces the cost of the mining operation.
Qualitative evaluation of extracted DNA on agarose gel approved the quality of extracted DNA as well as lack of fracture in all three methods.In general, several methods have been recommended for extraction of DNA from healthy plant, infected tissue and fungal pathogens, which usually require purification step for removing reaction inhibitors such as polysaccharides, phenolic materials or organic matter (Rollo et al. 1990). Existence of impurities in extracted DNA may prevents the proper cutting and attaching process, when it is required to digest the DNA by restriction enzymes and attach the adapters. But it does not seem it affects the successful implementation of PCR-based molecular assessment techniques, negatively. Effective method for extracting DNA from similar fungus such as Fusarium oxysporum is reported to be taken by cutting the fungus tissue, rubbing it with dry soil and special powder. In addition to this, rapid DNA extraction from F. oxysporum protocol is used by (GarcíaPedrajas et al. 1999). Effective method for extracting DNA genome of thefungi has been introduced byAl-Samarrai and Schmid (2000) through freezing and drying of mycelium.
NaOH extraction method (Wang et al. 1993) has been used for fast extraction of plant’s DNA. According to our knowledge, this method has not been used for extraction of DNA from Plant pathogenic fungi. The results showed that in addition to plants, the method could be used for extracting fungal DNA. Despite the small amounts of extracted DNA, the rate of operation is much higher than that of CTAB method. This could be so beneficial, particularly in the cases where there is no need for long-term preservation of DNA, as well as when early detection of the fungus is required.
Results of LAMP reactions by means ofthe DNA which was extracted viathree methods were positive, and at the completion point of reaction the cloudy state caused by magnesium pyrophosphate deposition could be observed (Figure 1). Also results ofelectrophoresis of reaction product and observation of a ladder pattern indicate that thereaction has been completed, successfully (Figure 1).
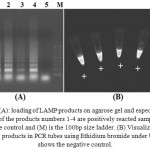 |
Figure 1a: loading of LAMP products on agarose gel and especial pattern of the products numbers 1-4 are positively reacted samples, 5 is negative control and (M) is the 100bp size ladder. (B) Visualization of LAMP products in PCR tubes using Ethidium bromide under UV. (-) shows the negative control.
|
No specific difference was observed among the DNA samples extracted via three methods in LAMP reaction. Despite the extremely low content of DNA in direct extraction from wood, and also in NaOH method, the LAMP reaction could easily amplify the parts of thefungus genome in an isothermal cycle. The results showed that the LAMP response is very sensitive toward detection and could detect very small values of target DNA in total DNA mixture, specifically.
Application of LAMP technique and direct isolation of the fungus Verticillium from wood can decrease the duration required for detection of pathogenic fungus in olive trees from about 20 days to less than half of a day.Simplicity is one of the advantages of this procedure, sensitivity and specialty of LAMP technique has been previously reported in the case of numerous pathogens such as potato leaf curl virus (Almasi et al. 2010) and cucumber mosaic virus (Ghasemi et al. 2010).
The results of this study about the fungus V. dahlia showed that regarding the high sensitivity of this method, we could probably detect the initial and hidden infections in olive wood. Using this method, it is also possible to detect the fungus pathogenic possibility in mother trees and this could be helpful in production of healthy and non-infected plants and control of contagion via plant propagation materials.
Acknowledgments
Different Steps of the research have been conducted in the biology lab of Agriculture and Natural Resources Research Center of Zanjan province and the cost of research funded referred to 108 issue of Agriculture Organization of Zanjan province as part of a special research project No. 91159-16-47-4.
References
- Azad H. A., Moeini M. R., Salati M And Moghadam S. A. M. Study on infection status of olive trees to fungal, bacterial and viral pathogens in different provinces of Iran. Final report of a research project. Plant Protection Institute. Tehran, Iran. 2000;120.
- Almasi M. A., Haghnazari A., Jafary H. Detection of the Potato Leaf Roll virus by using the RT-LAMP reaction. MSc. thesis in biotechnology. University of Zanjan. 2011.
- Al‐Samarrai T. H & Schmid J. A simple method for extraction of fungal genomic DNA. Letters in applied microbiology. 2000;30(1):53-56.
CrossRef - Atar L., Rahnam K., Araghi M. M., Sadravi M And Salati M. Reaction of olive cultivars to defoliating and to non-defoliating isolates of V. dahliae in Golestan province. Plant Protection Journal. 2009;24(4):406-412.
- Chen H. T., Zhang J., Sun D. H., Ma L. N., Liu X. T., Cai X. P., Liu Y. S. Development of reverse transcription loop-mediated isothermal amplification for rapid detection of H9 avian influenza virus. Journal of Virological Methods. 2008;151(2):200-203.
CrossRef - Fang X. E., Li J & Chen Q. One new method of nucleic acid amplification—loop-mediated is other mal amplification of DNA. Virologica Sinica. 2008;23(3):167-172.
CrossRef - Fu S., Qu G., Guo S., Ma L., Zhang N., Zhang S & Shen Z. Applications of loop-mediated isothermal DNA amplification. Applied biochemistry and biotechnology. 2011;163(7):845-850.
CrossRef - Fukuta S., Kato S., Yoshida K., Mizukami Y., Ishida A., Ueda J & Ishimoto Y. Detection of tomato yellow leaf curl virus by loop-mediated isothermal amplification reaction. Journal of virological methods. 2003;112(1):35-40.
CrossRef - Gadkar V & Rillig M. C. Evaluation of loop-mediated isothermal amplification (LAMP) to rapidly detect arbuscular mycorrhizal fungi. Soil Biology and Biochemistry. 2008;40(2):540-543.
CrossRef - García-Pedrajas M. D., Bainbridge B. W., Heale J. B., Pérez-Artés E & Jiménez-Díaz R. M. A simple PCR-based method for the detection of the chickpea-wilt pathogen Fusarium oxysporum f. sp. ciceris in artificial and natural soils. European Journal of Plant Pathology. 1999;105(3):251-259.
CrossRef - Ghasemi M., Haghnazari A., Jafary H. Detection of the Cucumber Mosaic Virus by RT-LAMP reaction. MSc thesis in biotechnology. University of Zanjan. 2011.
- Henson J. M., Goins T., Grey W., Mathre D. E & Elliott M. L. Use of polymerase chain reaction to detect Gaeumannomyces graminis DNA in plants grown in artificially and naturally infested soil. Phytopathology. 1993;83(3):283-287.
CrossRef - Imai M., Ninomiya A., Minekawa H., Notomi T., Ishizaki T., Tashiro M & Odagiri T. Development of H5-RT-LAMP (loop-mediated isothermal amplification) system for rapid diagnosis of H5 avian influenza virus infection. Vaccine. 2006;24(44):6679-6682.
CrossRef - Jafary H., Mehri N., Khoshkhati N and Bayati M. Role of biotic factors involved in olive decline in Tarom region. Abstract. Proceeding of the first national olive conference. Tehran. Iran. 2009;51.
- Mercado‐Blanco J., Rodríguez‐Jurado D., Pérez‐Artés E & Jiménez‐Díaz R. M. Detection of the nondefoliating pathotype of Verticillium dahliae in infected olive plants by nested PCR. Plant Pathology. 2001;50(5):609-619.
CrossRef - Moradi A., Haghnazari A. Evaluation of methods for detection of Salmonella. MSc thesis in biotechnology University of Zanjan. 2008;15753.
- Mori Y., Hirano T & Notomi T. Sequence specific visual detection of LAMP reactions by addition of cationic polymers. BMC biotechnology. 2006;6(1):3.
CrossRef - Nagamine K., Hase T & Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Molecular and cellular probes. 2002;16(3):223-229.
CrossRef - Niessen L & Vogel R. F. Detection of Fusarium graminearum DNA using a loop-mediated is other mal amplification (LAMP) assay. International Journal of Food Microbiology. 2010;140(2):183-191.
CrossRef - Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N & Hase T. Loop-mediated isothermal amplification of DNA. Nucleic acids research. 2000;28(12):e63-e63.
CrossRef - Okada K., Chantaroj S., Taniguchi T., Suzuki Y., Roobthaisong A., Puiprom O & Sawanpanyalert P. A rapid, simple, and sensitive loop-mediated isothermal amplification method to detect toxigenic Vibrio cholerae in rectal swab samples. Diagnostic microbiology and infectious disease. 2010;66(2):135-139.
CrossRef - Jurado D. R., Lopez B., Rapoport H. F & Diaz R. M. J. Present status of Verticillium wilt of olive in Andalucía (southern Spain). Bulletin OEPP (EPPO). 1993.
CrossRef - Rollo F., Salvi R & Torchia P. Highly sensitive and fast detection of Phoma tracheiphila by polymerase chain reaction. Applied microbiology and biotechnology. 1990;32(5):572-576.
CrossRef - Tomlinson J. A., Barker I & Boonham N. Faster, simpler, more-specific methods for improved molecular detection of Phytophthora ramorum in the field. Applied and Environmental Microbiology. 2007;73(12):4040-4047.
CrossRef - Usami T., Abiko M., Shishido M & Amemiya Y. Specific detection of tomato pathotype of Verticillium dahliae by PCR assays. Journal of general plant pathology. 2002;68(2):134-140.
CrossRef - Wang H., Qi M & Cutler A. J. A simple method of preparing plant samples for PCR. Nucleic acids research. 1993;21(17):4153.
CrossRef - Zhang X., Liao M., Jiao P., Luo K., Zhang H., Ren T & Cao W. Development of a loop-mediated isothermal amplification assay for rapid detection of subgroup J avian leukosis virus. Journal of clinical microbiology. 2010;48(6):2116-2121.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





