How to Cite | Publication History | PlumX Article Matrix
A Preliminary Investigation of Viral Pathogen Causing Foetal Abortion in Donkeys at Ethiopia
Uzoamaka Adaobi Okoli
Department of Biomedical Sciences, School of Health and Bioscience, University of East London, Stratford Campus, Water Lane, London E15 4LZ.
Corresponding Author E-mail: Uzoamaka.okoli@unn.edu.ng
DOI : http://dx.doi.org/10.13005/bbra/2542
ABSTRACT: Rabbit kidney (RK13) cells infected with virus isolated from a homogenate made from the placenta of aborted foetal donkeys were stained with Leishman’s stain, morphological changes including cytopathic effects (CPE), syncytia, and inclusion bodies were seen by light microscopy after incubation for 24hrs-96hrs at 370C. After 48hrs of incubation, about 60% of cells were infected. Another Set of RK 13 cells infected with either native virus or both ether treated virus and native virus in the presence of acyclovir was stained with Giemsa, morphology changes were observed in the native virus infected cell while little or no change was seen in infected cells in the presence of acyclovir and ether treated virus respectively. Virus infected RK13 cells were stained with acridine orange, intracellular fluorescent green colour was seen by fluorescence microscopy in the cell nucleus. The clinical history and CPE of the virus in RK13 cell are similar to Equine Herpes virus.
KEYWORDS: Acyclovir; Cytopathic Effects Fluorescence Microscopy; Rabbit Kidney Cells;
Download this article as:| Copy the following to cite this article: Okoli U. A. A Preliminary Investigation of Viral Pathogen Causing Foetal Abortion in Donkeys at Ethiopia. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Okoli U. A. A Preliminary Investigation of Viral Pathogen Causing Foetal Abortion in Donkeys at Ethiopia. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27740 |
Introduction
Donkeys are one of the major forms of rural transportation in Ethiopia. The donkey or ass, Equus africanus asinus is a domesticated member of the Equidae or horse family and many aspects of disease process in donkeys are similar to those of horses (Don., 2005). Many non-viral and viral pathogens are known to cause diseases in equids. Examples of non-viral are Rabies and Dourine while some examples of viral pathogen are African horse virus and Equine herpes virus (EHV). Among the above-mentioned pathogens. EHV is one of the major viral pathogen that usually causes disease outbreak with clinical sign and symptom: abortion, respiratory oedema, fever and neurological defect (Jin et al 2008).
The objective is to investigate the cause of an outbreak of disease which occurred in a number of the breeding modules that were recently stocked with donkey brought from two organisations in the UK and USA which included five new stud males. The disease lead to decreased production in foals since abortion rates increased form 10% to almost 70%.
Materials and Methods
Viruses and Cells
The virus and RK13 cells were kindly provided by Dr Sally Cutler, University of East London. Samples were made from placenta homogenate of aborted foetal donkeys in Ethiopia. Virus was propagated in rabbit kidney cells (RK13). RK13 cells grew as adherent cell line on the surface of culture flask. Cells were observed with phase contrast microscopy to check that cells were confluent. Suspensions were prepared by dispersing RK13 cells in 8ml fresh medium and incubated at 37oC. The fresh culture medium consists of 5% foetal calf serum (FCS), since the culture flasks contained about 5×104 cells the volume of 5% FSC (950µl) to make up 1ml was calculated. 100µl of the filtrate (placenta homogenate) was inoculated into the RK13 cells and incubated for 24hrs-96hrs in a CO2 incubator at 370C as described by Davis (2002).
Cell Cultivation
To establish the presence of fungi, bacteria and protozoa, a small amount of the placenta homogenate was streaked onto two blood agar plates (kindly provided by Dr Sally Cutler, UEL). One was incubated aerobically and the other anaerobically at 370C
Staining Methods
Leishman’s Stain
A slight modification of Rooney’s techniques (Davis, 2002) was applied to RK13 cells. Cells were stained with 500µl of Leishman’s stain and differentiated using 500µl of buffered water of pH 6.8. There were incubated at 370C. The cells were examined and counted under a light microscope using Immersion oil.
Giemsa Stain
To ascertain the number of infected units, RK13 cells infected with native virus, ether treated virus and native virus in the presence of Acyclovir, were individually fixed with 500µl of Industrial methylated spirit (IMS) and rinsed with 500µl of phosphate buffered saline (PBS). RK13 cells were stained with 0.05 Giemsa stain. Stain was removed with 500µl of buffered water as described by Celis , (1998).
Acridine Orange Stain
To identify the viral nucleic acid, RK13 cells infected with virus was fixed with 500µl of 2.5% of glutaraldehyde, rinsed with 500µl of buffered water and stained with 500µl of acridine orange. The stain was removed with Walpole’s buffer of pH 4.2. (Lauretti, 2009)
Result
Identification of Non-Viral Pathogens
No growth was observed from both the aerobic and anaerobic incubated blood agar indicating the absence of non-viral pathogens.
Evidence of Viral Activity
RK13 cells nucleus stained red while the cytoplasm stained blue with Leishman’s stain. The cells in the control and infected RK13 cells were counted (Figure 1). The virus infected RK13 cells developed morphological changes as follows: after 24 hrs of incubation, 10% of the cells had CPE , some cells had normal shape and some lost their structures due to cell lysis. Within 48hrs 60%-80% of the cells developed syncytia (multinucleated giant cells), cell lysis, swollen cells and intranuclear inclusion bodies which indicated viral activity.
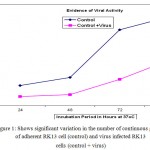 |
Figure 1: Shows significant variation in the number of continuous growth of adherent RK13 cell (control) and virus infected RK13 cells (control + virus)
|
Effect of Acyclovir and Ether on Virus Infected RK13 Cells
RB13 cells stained bluish purple (basophilic) with Giemsa stain. Round, swollen cells in clusters like bunch of grapes, Cell fusion, cell lysis and shape loss were observed in virus infected RK13cells. RK13 cells infected with ether treated virus had 10% swollen and cell lysis while RK13 cells treated with virus in the presence of acyclovir had 5% of round swollen cells and cell lysis.
Determination of Nucleic Acid
RK13 cells (control) appeared to be normal and did not fluorescence while virus infected RK13 cells did fluorescence.
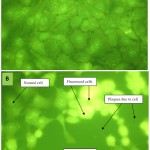 |
Figure 2: Comparison of fluorescence microscopy of both normal RK13 cells (A) and virus infected RK13 cells (B) at 100 x magnification
|
Discussion and Conclusion
Figure 1 appears to be consistent with the finding that CPE were well developed within 48hrs. It can be seen that intracellular infectious units increased exponentially until a peak was reached at 80- 96hrs with a high yield infectious cell unit. Enders (1954) suggested categorizing viruses into classes of those that induced cell disintegration; inclusion bodies formation and cell disintegration; formation of syncytia (multinucleated cells) and cytopathic effects, (CPE). The morphological changes result of virus causing high morbidity and mortality in donkeys is similar to veterinary herpes virus as categorised by Enders. Acridine orange interacts with Ribonucleic acid (RNA) and Deoxyribonucleic acid (DNA) by electrostatic attraction, emitting red light or interpolation, emitting green light respectively under ultraviolet light (Lauretti.,2003). This agrees with the virus characteristic appearance of fluorescent green colour (Figure2) as EHV is a DNA virus. Equine herpes virus are sensitive to ether and acyclovir; they significantly reduced DNA replication hence less viral multiplication and the number of plaque and plaque size. Although acyclovir has been found to be active against a small number of veterinary herpes virus in cell culture significant in vivo activity has been demonstrated for acyclovir only against EHV.1 in hamster model (Garre et al, 2007; Collins, 1983).
Further serologic studies are needed to identify the particular strain of this EHV virus. However, by correlating viral inclusion identification, clinical history, physical finding and putting into consideration that 5 mare were introduced 18 months prior to the outbreak, as previously reported outbreaks were preceded by introduction of new animal (Jin et al, 2008; Pusterla et al, 2009; Walter, J., et al 2013). There should be procedures put in place like restriction of foreign contact prior to vaccination of newly introduced mare. From my result, I document that the virus is suspected to be EHV and acyclovir displayed a better activity against the equine virus infection in vitro without cell viability.
Acknowledgment
I wish to thank Dr Sally Cutler, Dr Claudio Scotti and Poonam Tripathi of University of East London for their generous support and for making this work possible.
Conflict of Interest
None
Funding Source
All reagents and material of study were of Sigma -Aldrich (UK) and was kindly provided by the Department of Biomedical Science, University of East London, School of East London.
References
- Celis J. E. (ed)) Cell Biology: A Laboratory Handbook, 2nd edn. San Diego. 1998;40-47.
- Collins P. Journal of Antimicrobial Chemotherapy. The spectrum of antiviral activities of acyclovir in in vitro and in vivo. J Antimicrobial Chemotherapy. 1983;12:19–27.
CrossRef - Davis J. M. Basic cell culture, Practical Approach, IRL 2nd Oxford, UK. 2002;203-208.
- Don E. W & DeeAnn M. R., ed . Equus asinus. Mammal Species of the World. A Taxonomic and Geographic Reference (3rd ed.). Johns Hopkins University Press. 2005.
- Ender J. F. Bell J. A., Dingle J. H., Francis T., Hilleman M. R., Huenbner R. J., Payne M. M. A. Adenovirus Group name proposed for new respiratory tract viruses. Science. 1956;20 24. (3212):119-120.
- Garre B. K., Meulen V. J., Nugent J., Neyts S., Roubels P., Backer H. In vitro susceptibility of six isolated of equine HV1 to acyclovir gauciclovir, cidofovir adefovir, PMEDAP and foscarnet. Veterinary microbiology. 2007;122:43-51.
CrossRef - Jin L., Valentine B. A., Baker R. J., Lohr C. V., Gerlach R. F., Bildfell R. J., Moerdyk-Schauwecker M. An outbreak of fatal herpesvirus infection in domestic rabbits in Alaska. Vet Pathol. 2008;45(3):369-74.
CrossRef - Lauretti. Use of acridine orange staining for detection of rotavirus RNA in polyacrylamide gels. Journal of virological methods. 2003:114:29-35.
CrossRef - Pusterla N., Mapes S., Madigan J. E., Maclachlan N. J., Ferraro G. L., Watson J. L., Spier S. J., Wilson W. D. Prevalence of EHV-1 in adult horses transported over long distances. Vet Rec. 2009;165:473–475.
CrossRef - Walter J., Seeh C., Fey K., Bleul U., Osterrieder N. Clinical observations and management of a secere equine herpes virus type 1 outbreak with abortion and encephalomylities. Acta Vet Scand. 2013;5(55):19.

This work is licensed under a Creative Commons Attribution 4.0 International License.





