How to Cite | Publication History | PlumX Article Matrix
Assessment of Phytochemical Constituents and Antimicrobial Activity of Lantana Camara L.
Ajay Kumar1, Shikha Singh1 and Pragati Saini2
1Department of Life Sciences, ITM University, Gwalior (MP) India.
2Department of Microbiology, Kamla Raja Girls PG College, Gwalior (MP), India.
Corresponding Author E-mail: kumarajayitm@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2531
ABSTRACT: In present study the phytochemical constituents such as total phenol, total flavonoid contents and antibacterial activity against four gram negative and two gram positive isolates Escherichia coli, Pseudomonas aeruginosa, Enterobacter aerogens, Proteus vulgaris, Lactobacillus, S. aureus, Bacillus subtilis and antifungal activity against four fungal strains Penicillium, Aspergillus niger, T. mentagrophytes, Microsporum fulvum of petroleum ether, diethyl ether, chloroform and acetone extract of leaves and flowers of Lantana camara L were evaluated. Maximum zone of inhibition was recorded in the presence of free flavanoid fraction of the plant extract against Trichophyton mentagophytes and Microsporum fulvum which was the most susceptible fungus for all the extracts tested. The extract also compared favourably with streptomycin which serves as a positive control. Minimum inhibitory concentration (MIC) was recorded for all bacteria and fungi in which highest MIC was of B. subtilis and M. fulvum. The UV-Vis and FTIR spectroscopic analysis also revealed the presence of different active groups and bonds. L. camara contains phytochemical compounds with antibacterial & antifungal activities. Moreover, the chloroform & acetone leaf & flower extracts of L. camara are active against pathogenic microorganisms.
KEYWORDS: Antimicrobial Activity; Insecticidal; Larvicidal; Lantana Camara and Verbenaceae
Download this article as:| Copy the following to cite this article: Kumar A, Singh S, Saini P. Assessment of Phytochemical Constituents and Antimicrobial Activity of Lantana Camara L. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Kumar A, Singh S, Saini P. Assessment of Phytochemical Constituents and Antimicrobial Activity of Lantana Camara L. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27801 |
Introduction
In ancient times, plants have been utilized as an important source of medicines as they are a reservoir of chemical agents with antimicrobial properties. Medicinal plants, which form the backbone of traditional medicine, in the last few decades, have been the subject for very intense pharmacological studies. Lantana camara L., a member of family Verbenaceae, is an evergreen, aromatic weed, native to tropical America, but it is now cultivated in many other parts of the world (Raghu et al., 2004). Almost all parts of this plant have been used traditionally for treatment of several ailments due to their multiple biological activities such as antihelmintic (Patel et al., 2011), anti-leukemia (Badakhshan et al., 2009), larvicidal (Kumar & Maneemegalai 2008), antioxidant (Bhakta & Ganjewala, 2009), antibacterial, antiproliferative (Gomes-de Melo et al., 2010), antiulcerogenic (Thamotharan et al., 2010), haemolytic (Kalita et al., 2011), antimutagenic activity, antihypertensive (Kaur et al., 2010) and hepatoprotective activities (Abou El-Kassem et al., 2012). Most importantly, the flower extracts of L. camara are used in folk medicine for the management of several disorders including cancers, asthma, tumors, bilious fevers, chicken pox, eczema, measles, ulcers, swellings, high blood pressure, catarrhal infections, rheumatism, tetanus, malaria and abdominal viscera (Ghisalberti, 2000; Day et al., 2003). L. camara L. is a flowering ornamental plant belonging to family verbenaceae. L. camara also known as lantana, wild sage, Surinam tea plant, Spanish flag and West Indian lantana. L. camara is a well known medicinal plant in traditional medicinal system. L. camara contains lantadenes, the pentacyclic- triterpenes which is reported to possess a number of useful biological activities.
Recently, there is a revival of interest in the use of plants as natural remedy for medication of several health disorders due to the reason that they possess multiple biological activities, compatibility with system biology, potential physiological functions and protective role against several degenerative diseases (Suhaj, 2006; Tadhani et al., 2007, Espin et al., 2007; Wolfe et al., 2009; Lifschitz, 2012; Gawe, 2012). The extraction of antioxidant components from a plant material is a crucial step so as to accomplish further fractionation, isolation, purification and characterisation of biologically active compounds. A variety of extraction techniques such as orbital shaker, stirring, accelerated solvent extraction, microwave assisted extraction and supercritical fluid extraction etc., are in use to recover antioxidant and nutraceutical components from plants. All techniques have some advantages and disadvantages over others, but none of these is claimed to be perfect in all aspects. In view of the above-mentioned reports, this study was planned to explore the availability of potent antimicrobial agents of L. camara flowers using different solvents.
Materials and Methods
Collection of Plant Materials and Test Organisms
The plant materials used are leaves and flower of L. camara Linn. The samples were collected, at Turari campus, ITM University, Gwalior (MP) India.
The microbial cultures used in this study, were Escherichia coli (MTCC 40), Pseudomonas aeruginosa (MTCC 8165), Bacillus subtilis (MTCC 1143), Lactobacillus sp., Proteus vulgaris (MTCC-1771), S. aureus (MTCC 3160), Enterobacter aerogens (MTCC-7325) Penicillium sp., Aspergillus niger (MTCC 9652), T. mentagrophytes (MTCC 7687) and Microsporum fulvum (MTCC 2837). These cultures were procured from Microbial Type Culture Collection Centre, Chandigarh. Bacterial isolates were inoculated into Nutrient broth and fungus into potato dextrose agar then incubated at 37°C for 24 hours and at 27°C for 3-5 days for fungi respectively. Penicillin antibiotic (1 mg/ml) was used as positive control for the test bacterial strains. Sterilized distilled water and dimethyl sulfoxide (DMSO) were used as negative control.
Processing of Plant Materials
The leaves and flowers of L. camara L were dried in the hot air oven at 40oC until all the water contents dried off. The dried samples were ground separately into fine powder. About 180 g each of the powdered specimen was soaked in the solvents of acetone, chloroform, petroleum ether, diethyl ether by using soxhlet extraction method for the extraction of pure solvent until the colour of solvent became white.
Antimicrobial Sensitivity of Crude Extract of Leaves and Flowers of Lantana Camara L. Antibacterial Sensitivity Test by Well Agar Diffusion Method
The crude extract was screened for antimicrobial activity using well agar diffusion method as described by Russell & Furr (1977). Muller Hinton Agar (MHA) was prepared in Mac. Carthney bottle and cooled to 45°C and inoculated with loopful culture. MHA medium was poured into a well-labelled Petri dish and allowed to set. Holes or wells were then bored into the set inoculated MHA using sterile cork borer. Using sterile syringe, extracts at concentration of 25 mg/ml were transferred aseptically into the wells. The plates were left on the bench for about one hour to allow proper diffusion of the extract into the MHA. This procedure was repeated for each bacterial isolate. The plates were incubated right way up at 37°C for 24 hours. After 24 hours the plates were observed for clear zone of inhibition, which indicate the relative susceptibility of the bacteria to the extract. The diameter of the zones of inhibition was measured and recorded in mm.
Antifungal Sensitivity Test by Biomass Reduction Method
The crude extract was screened for antifungal activity using biomass reduction method. The medium used was Sabouraud’s dextrose (SD) broth. 50 ml of each flask containing sterile molten SD broth was inoculated with a loopful culture with four different extracts of leaves, flower and also antifungal agent (cycloheximide) and kept in incubator shaker at 270 C for 48 hrs. After 48 hrs, the broth culture was filtered through pre-weighted Whatman filter paper. Filter paper was dried inside an oven at 50oC for overnight. After drying, the biomass was further weighted and percentage reduction in biomass was calculated using the following formula
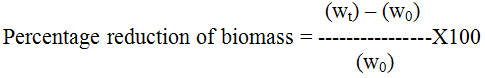
Where, w0 is Initial wt. of filter paper and wt final wt. of filter paper
Phytochemical Analysis of Crude Extracts
Phytochemical screening of the L. camara was performed to detect the presence of different classes of constituents, such as alkaloids, steroids, flavanoids, reducing sugar and tannin (Naz, 2013).
Thin Layer Chromatographic Analysis
Each extract was checked by Thin Layer Chromatography (TLC) on analytical plates over silica gel (TLC-grade; Merck India). For each extract of leaf and flower of L. camara one solvent system was used as developing systems as petroleum ether: chloroform: water (6:2:2) with boiling point 40-60oC. In each case, the spots were visualized by exposure of plates to iodine vapour.
Instrumental Analysis
UV-Vis Spectrophotometer Analysis
The extracts were examined under visible and UV light for proximate analysis. For UV-Vis spectrophotometer analysis, the extracts were centrifuged at 3000 rpm for 10 min and filtered through Whatmann No. 1filter paper. The sample is diluted to 1:10 with the same solvent. The extracts were scanned in the wavelength ranging from 200-1100 nm using Perkin Elmer Spectrophotometer and the characteristic peaks were detected.
Fourier Transforms Infrared Spectrophotometer (FTIR) Analysis
Fourier Transform Infrared Spectrophotometer (FTIR) is the most powerful tool for identifying the types of chemical bonds (functional groups) present in compounds. The wavelength of light absorbed is characteristic of the chemical bond as can be seen in the annotated spectrum. By interpreting the infrared absorption spectrum, the chemical bonds in a molecule can be determined. 10 mg of the dried extract powder was encapsulated in 100 mg of KBr pellet, in order to prepare translucent sample discs. The powdered sample of each plant specimen was loaded in FTIR spectroscope (Shimadzu, IR Affinity, Japan), with a scan range from 400 to 4000 cm-1 with a resolution of 4 cm-1.
Results
Antibacterial Activity of L. Camara Leaf Extract
Figure 1 shows that L. camara leaf extracts have strong antibacterial activity. Extracts were prepared in petroleum ether, diethyl ether, chloroform and acetone. However only chloroform extract was found to be the most effective against all the bacteria except Pseudomonas and E. coli.
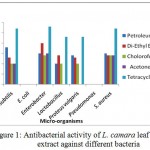 |
Figure 1: Antibacterial activity of L. camara leaf extract against different bacteria
|
Antibacterial Activity of L. Camara Flower Extract
L. camara flower extracts also possess strong antimicrobial activity. The extracts were prepared in petroleum ether, di ethyl ether, chloroform and acetone. However only chloroform extract was found to be the most effective against all the bacteria except E. coli and Pseudomonas.
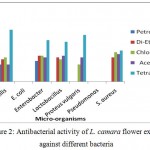 |
Figure 2: Antibacterial activity of L. camara flower extract against different bacteria
|
Percentage Inhibition of Fungal Biomass Using Leaf Extracts of L. Camara
L. camara leaf extracts possess strong antifungal activity. The extracts were prepared in petroleum ether, diethyl ether, chloroform and acetone. Only acetone was found most effective against Aspergillus niger whereas in case of Penicillium, the chloroform showed the poor activity. Maximum effectiveness was also observed with acetone against T. mentagrophytes. The plant extract showed the more effective result as compared to cyclohexamide antibiotic.
 |
Figure 3: Percentage inhibition of fungal biomass using leaf extract of L. camara
|
Percentage Inhibition of Fungal Biomass Using Flower Extract of L. Camara
L. camara flower extracts possess strong antifungal activity. The extracts were prepared in petroleum ether, diethylether, chloroform and acetone. However, only petroleum ether found to be the most effective against all the tested fungi.
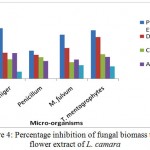 |
Figure 4: Percentage inhibition of fungal biomass using flower extract of L. camara
|
Preliminary Phytochemical Screening
Qualitative test detected around five common secondary metabolites in four different extracts of leaf and flower of L. camara such as alkaloid, and other minor compounds like soluble starch, flavanoid test, tannins, reducing sugar, alkaloid however could not be detected in flower and reducing sugar could not be detected in leaf (Table 1).
Table 1: Phytochemical analysis of extracts of Leaves and Flower of L. camara
| Phytochemicals | Leaf | Flower | |||||||
| P. ether | Diethyl ether | Chloroform | Acetone | P. ether | Diethyl ether | Chloroform | Acetone | ||
| Alkaloid | +ve | -ve | -ve | -ve | -ve | -ve | -ve | -ve | |
| Reducing Sugar | -ve | -ve | -ve | -ve | +ve | -ve | -ve | -ve | |
| Flavanoid | +ve | -ve | -ve | -ve | +ve | -ve | -ve | -ve | |
| Soluble Starch | +ve | -ve | -ve | -ve | -ve | +ve | -ve | +ve | |
| Tanin | -ve | -ve | -ve | +ve | -ve | -ve | -ve | +ve | |
TLC Analysis of Leaves and Flower Crude Extracts of L. Camara
The TLC chromatogram of chloroform extracts of both flower and leaf is presented in Table 2. The spots were characterized by RF value and colour were visualised after spraying with iodine vapour. In case of chloroform extracts of leaves the RF value is 0.45 and the colour of the spot is yellow which shows the presence of flavanoid-glycoside compound.
Table 2: Thin layer chromatogram of crude extracts of L. Camara
| Extract | Solvent system | Result |
| Chloroform extracts of leaves of L. camara | Petroleum ether : chloroform : water – 6:2:2 | 0.45 |
| Chloroform extracts of flower of L. camara | Petroleum ether : chloroform : water – 6:2:2 | 0.54 |
Minimum Inhibitory Concentration (MIC) of the Chloroform Solvent Extract
MIC of the chloroform extract showed in Table 3 for the test bacterial cultures which reveal that the MIC value is 0.195 mg/ml for Gram negative bacterial culture except E. coli while no MIC was recorded against S. aureus.
MIC of the chloroform extract showed in Table 4 for the test a fungus culture, which is 0.390 mg/ml for A. niger & Penicillium species.
Table 3: Minimum inhibitory concentration (MIC) of chloroform extract of L. camara on test bacterial cultures
| Bacteria isolates | MIC (mg/ml) | |
| Leaf Extract | Flower extract | |
| B. subtilis | 3.125 | 3.125 |
| S. aureus | 0 | 0 |
| E. coli | 0 | 0 |
| Enterobacter aerogens | 0.195 | 0.195 |
| Proteus vulgaris | 0.195 | 0.195 |
Table 4: Minimum inhibitory concentration (MIC) of chloroform extract of L. camara on test fungus cultures
| Fungal isolates | MIC(mg/ml) | |
| Leaf Extract | Flower extract | |
| Aspergillus niger | 0.390 | 0.390 |
| Penicillium species | 0.390 | 0.390 |
| M. fulvum | 0.781 | 0.781 |
| T. mentagrophytes | 0.485 | 0.431 |
UV-Vis and FTIR Spectrum for the Chloroform Leaf Extract of L. Camara
Chloroform extracts of leaf of L. camara exhibited a characteristic band at 1084cm-1 indicating the presence of (OH group) and at 1389.34cm-1 (C=H group) and 1465.05cm-1 for (C-H stretching) and 17.34.99 cm-1 for (C=O carbonyl group) 2918.99cm-1 for (OH group) and 3553.78cm-1 for (OH group) (Figure 5a & b).
 |
Figure 5a.b: UV-Vis & FTIR spectrum for the chloroform leaf extract of L. camara
|
UV-Vis and FTIR Spectrum for the Chloroform Flower Extract of L. Camara
The extracts of flower of L. camara exhibited a characteristic band at 1405.45 for (C-H stretching) and 1739.85 cm-1 for (C=O keto) and 2822.40 cm-1 for (C-H stretching) and 2853.34cm-1 for (C-H stretching) and 33402 cm-1 for (OH group) (Figure 6a & b).
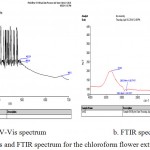 |
Figure 6a.b: UV-Vis & FTIR spectrum for the chloroform flower extract of L. Camara
|
Discussion
In present work the biochemical composition of L. camara flower and leave extracts were studied. However, the biochemical as well as the chemical compositions of the concerned plants parts is often influenced by different origins, environmental and seasonal factors. Previously reported seasonal changes in the chemical composition of essential oils in more than seventy L. camara from different parts of the world. Very recently, Bhakta and Ganjewala (2009) reported the effects of leaf position on the level of secondary metabolites in L. camara. These studies have clearly suggested the geographical, developmental stage of the plant and or biochemical compositions in L. camara.
L. camara has been studied extensively for their antibacterial properties (Mello et al., 2005; Verma & Verma, 2006). L. camara possess many important biological activities. Lantadenes present in all L. camara is believed to be responsible for almost all the biological activities (Barre et al., 1997). In addition, other secondary metabolites such as alkaloids, terpenoids, and phenolics could be held partially responsible for some of these biological activities (Barre et al., 1997). However, constituents like 1, 8-cineole, sabinene, and caryophyllene and other minor constituents viz., E-nerolidol, bicyclogermacrene, and pinene identified in leaf essential oils were also found to be responsible for the biological activities of essential oils (Sonibare & Effiong, 2008). Therefore, antibacterial activities of L. camara leaf and flower extracts reported here might be due to the presence of some of these chemical constituents particularly lantadenes and theveside in the extracts.
Though, the mechanism of the action of these chemical constituents is not yet fully known it is clear that the effectiveness of the extracts largely depends on the type of solvent used. Perhaps it is one of the reasons behind differences in the antibacterial activities of the plants. These differences in the susceptibility of the test organisms to the different extracts might be due to the variation in the rate at which active ingredients penetrate their cell wall and cell membrane structures. In conclusion, L. camara plant with flower & leaf extracts have displayed variable antibacterial & antifungal activities most probably due to the differences in the biochemical and phytochemical composition.
The extracts of L. camara exerted a broader spectrum of inhibitory activity on Gram positive bacteria than Gram negative. However, Staphylococcus aureus was found to be resistant to the extract (Ganjewala et al., 2009). These extracts of leaves and flower also show activity against fungus and dermatophytes. Chloroform extract of leaves and flower shows highest activity against fungus and dermatophytes.
Phytochemical screening helps to reveal the chemical nature of the constituents of the plant extracts and the one that predominates over the others (Enwuru et al., 2008). It may also be used to search for bioactive lead agents that could be used in the partial synthesis of some useful drugs. Phytochemical screening of L. camara leaf extracts revealed the presence of alkaloid, tannins, flavonoids and soluble starch as major active secondary metabolite while reducing sugar were absent but the extracts of flower of L. camara revels the presence of reducing sugar, tannins, flavanoid and soluble starch while alkaloid was absent. This is similar to phytochemical study of aerial parts of Lantana camara. Many researchers have also shown the presence of flavonoids in the leaf and flower extract of L. camara (Sathish & Maneemegalai, 2008).
Conclusion
The ultimate conclusion of this study supports the traditional medicine use of different plant extracts in treating different infections caused by pathogenic bacteria & fungi in India. This study showed that L. camara contains phytochemical compounds with antibacterial & antifungal activities. Moreover, the chloroform & acetone leaf & flower extracts of L. camara are active against pathogenic tested microorganisms. It also suggests that a great attention should be paid to medicinal plants which are found to have plenty of pharmacological properties that could be sufficiently better when considering a natural food and feed additives to improve human and animal health.
Acknowledgements
The authors would like to thank to Head, Department of Life Sciences for providing lab facility and also grateful to DST-SERB, New Delhi for financial assistance.
References
- El-Kassem L. T. A., Mohammed R. S., El-Souda S. S., El- Anssary A. A., Hawas U. W., Mohmoud K and Farrag A. R. Di galacturonide flavones from Egyptian Lantana camara flowers with In Vitro antioxidant and In Vivo hepatoprotective activities. Z Naturforsch C. 2012;67(7-8):381-90.
CrossRef - Badakhshan M. P., Sreenivasan S., Jegathambigai R. N and Surash R. Anti-leukemia activity of methanolic extracts of Lantana camara. Res. 2009;1:274-279.
- Barre J. T., Bowden B. F., Coll J. C., Fuente D., Janairo V and Rajasa C. Y. A bioactive triterpenoid from Lantana camara. Phytochem. 1997;4:321-324.
CrossRef - Bhakta D and Ganjewala D. Effect of Leaf Positions on Total Phenolics, Flavonoids and Proanthocyanidins Content and Antioxidant Activities in Lantana Camara (L). Journal of Sci. Res. 2009;1(2):363-369.
- Enwuru N. V., Ogbonna S. O., Nkemehule F., Enwuru C. A and Tolani O. Evaluation of antibacterial activity and acute toxicity of the hydroethanolic extract of Stachytarpheta angustifolia (Mill).Vahl. African J. Biotechnol. 2008;7(11):1740-1744.
CrossRef - Espin J. C., Garcia-Conesa M. T and Barberan F. A. Nutraceuticals facts and fiction. Phytochem. 2007;68:2986-3008.
CrossRef - Ganjewala D., Sam S and Khan K. H. Biochemical compositions and antibacterial activities of Lantana camara plants with yellow, lavender, red and white flowers. Eur Asia J Biol Sci. 2009;3:69-77.
CrossRef - Gawe E. Chemical composition of lucerne leaf extracts and its applications as a phytobiotic in human nutrition. Acta Sci Pol Technol Aliment. 2012;11(3):303-310.
- Ghisalberti E. L. Lantana camara (Verbenaceae). Fitoterapia. 2000;71(5):467-486.
CrossRef - Gomes-de-Melo J., Sousa-de-Araujo T. A., Nobre-de-Almeida T. C., Lyra-de-Vasconcelos C. D., Desterro-do- Rodrigues M and Carneiro-do-Nascimento S. Antiproliferative activity, antioxidant capacity and tannin content in plants of semi-arid Brazil. Molecules. 2012;15:8534-8542.
CrossRef - Kalita S., Kumar G., Karthik L and Rao K. V. Phytochemical composition and In-Vitro haemolytic activity of Lantana camara (Verbenaceae) leaves. Pharmacol News letter. 2011;1:59-67.
- Kaur S., Kumar S., Kaur P and Chandel M. Study of antimutagenic potential of phytoconstituents isolated from Terminalia arjuna in the Salmonella/Microsome Assay. J. Biomed. Sci. 2010;2:164-177.
CrossRef - Kumar M. S and Maneemegalai S. Evaluation of larvicidal effect of Lantana camara Linn against mosquito species Aedes aegypti and Culex quinquefasciatus. Ad Biol Res. 2008;2:39-43.
- Lifschitz C. New actions for old nutrients. Acta Sci. Pol. Technol. Aliment. 2012;11(2):183-192.
- Mello F. B., Jacobus D., Carvalho K and Mello J. R. B. Effects of Lantana camara (Verbenaceae) on general reproductive performance and teratology in rats. Toxicol. Res. 2005;45:459-466.
CrossRef - Naz R and Asghari B. Phytochemical screening, antioxidants and antimicrobial potential of Lantana camara in different solvents. Asian Pac. J. Trop. Dis. 2013;3(6):480-486.
CrossRef - Patel J., Kumar G. S., Deviprasad S. P., Deepika S and Qureshi M. S. Phytochemical and antihelmintic evaluation of Lantana camara (L.) var. aculeate leaves against Pheretima posthuma. J. Global Trends Pharm. Sci. 2011;2:11-20.
- Raghu C., Ashok G., Dhanaraj S., Suresh B and Vijayan P. In vitro cytotoxic activity of Lantana camara Linn. Indian J. Pharmacol. 2004;104(3):1106-1114.
- Russell A and Furr N. Antibacterial activity of a new chloroxylenol preparation containing ethylene diamine tetra acetic acid. J. Appl. Bacterial United Kingdom. 1977;43:253.
- Sathish M and Maneemegalai S. Evaluation of larvicidal effect of Lantana camara L against mosquito species Aedes aegypti and Culex quinquefasciatus. Adv. Biol. 2008;2:39-43.
- Sonibare O and Effiong I. Antibacterial activity and cytotoxicity of essential oil of Lantana Camara leaves from Nigeria. African J. Biotechnol. 2008;7(15):2618-2620.
- Suhaj M. Spice antioxidants isolation and their antiradical activity. Food Composit. Anal. 2006;19:531-537.
CrossRef - Tadhani M. B., Patel V. H and Subhash R. In-vitro antioxidant activities of Stevia rebaudiana leaves and callus. Food Compost. Anal. 2007;20:323-329.
CrossRef - Thamotharan G., Sekar G., Ganesh T., Sen S., Chakraborty R and Kumar S. N. Antiulcerogenic effects of Lantana camara leaves on In Vivo test models in rats. Asian J. Pharm. Clinical Res. 2010;3:57-60.
- Verma R. K and Verma S. K. Phytochemical and termiticidal study of Lantana camara aculeata leaves. Fitoterapia. 2006;77(6):466-468.
CrossRef - Wolfe A. R., Ogbonna E. M., Lim S., Li Y and Zhang J. Dietary linoleic and oleic fatty acids in relation to severe depressed mood: 10 years follow-up of a national cohort. Neuro-Psychoph. 2009;33(6):972-977.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





