How to Cite | Publication History | PlumX Article Matrix
G. Sai Vaishnavi and D. Muralidhara Rao
Department of Biotechnology Sri Krishnadevaraya University, Ananthapuramu, India.
Corresponding Author E-mail: Saivaishnavig@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2544
ABSTRACT: The present paper deals with some of those highlighting biochemical capabilities, that lay foundation to exploit the organism ‘caldicellulosiruptor saccharolyticus’emphasizing for finding substitutes for fossil fuels as the organism bears all hallmarks of being adopted as a ‘future biofuel producer’. Belonging to the genus ‘Caldicellulosiruptor’, it is one of the most well studied thermophilic bacterium that possess unique biological chemistry in fermenting substrates very easily with its efficient metabolism.
KEYWORDS: Caldicellulosiruptor Saccharolyticus; Biofuel; Metabolism
Download this article as:| Copy the following to cite this article: Vaishnavi G. S, Rao D. M. Biological Chemistry of Extreme Thermophile “Caldicellulosiruptor Saccharolyticus”- A Future Biofuel Producer. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Vaishnavi G. S, Rao D. M. Biological Chemistry of Extreme Thermophile “Caldicellulosiruptor Saccharolyticus”- A Future Biofuel Producer. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27591 |
Introduction
A biofuel is a fuel from the biological source using agriculture and bacterial strains. The primary source is the photosynthesis leading to the formation of carbohydrates and carbon manifestation leading to fuel in different forms such as solid, liquid and gas forms. The three forms of biofuels are derived such as perennial crops, sugar cane and corn strach.1,2 Biofuels are often classified as first, second and third generation biofuels (Table 1).
Table 1: Classification of Biofuels 28
| Biofuels | First generation biofuels | Second generation biofuels | Third generation biofuels |
| Features
Examples |
Fuels produced from materials in food industry.
Biodiesel from starch derived biogass |
Fuels produced from waste products
Bioethanol and biohydrogen from agricultural waste |
Fuels produced using aquatic microbes
Biodiesel from algal source |
First generation biofuels utilize food crops as feed stock for biofuel production, while second generation biofuels utilize non-food biomass. Third generation biofuels use algae and microbes as fuel source materials.3 Biofuels are getting significiant attention, worldwide due to the depletion of fossil fuels and concerns regarding climate change. They are recognized for their potential role in reducing green house gas (GHG) emissions and providing energy security (Fig 1). The production of biofuels are not at all recommended. It is expensive to convert raw materials of food crops in to fuels; due to food crisis in many countries. Therefore, the scientists have turned their attention in employing the microbes in biofuel production, with an expectation that that may be the future biofuel producers.
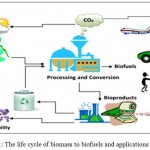 |
Figure 1: The life cycle of biomass to biofuels and applications 29
|
Speciality of Genus “Caldicellulosiruptor”
The organism belonging to genus ‘Caldicellulosiruptor’ are gram positive, thermophilic, anaerobic, cellulolytic and with low G+C content. They are known to possess extreme capability in degrading the plant biomass and also carbohydrates that are complex in nature. The bacteria belonging to the genus has a speciality due to their thermophilic nature. Among the genus, Caldicellulosiruptor saccharolyticus is known to contain 94.4 to 96.6 % rRNA identity, eith different metabolic approach in degrading the complex substrates in their environment,4 promoting itself as plant-biomass degrading genus for biofuel production.
Ideal Characteristics of Biofuel Producer – “Caldicellulosiruptor Saccharolyticus”
Since the members of this genus possess certain interesting characteristics, providing a scope for biofuel productions in the future. Therefore this particular organism is of worth study.
The capabilities possessed by the organism, that makes it ideal for a set industrial application with a ray of hope on biofuel production.
Interspecies Interaction of “Caldicellulosiruptor Saccharolyticus” Boosting Product Yield
Distinct microbial populations in the environment of natural resources like air, water, soil interact in a wide range with beneficial and mutualistic effects.
Communities of different microbial populations can be built for tuning the metabolic constitution carefully for targeting new functiuons and also for the study of different interactions.5
The extreme thermophiles ‘Caldicellulosiruptor saccharolytcus’ DSM 8903 and ‘Caldicellulosiruptor kristjanssoni’ DSM 12137 when combined in a co=culture, the cell free growth supernatant of C.saccharolyticus enhanced the growth of C.kristjanssoni in batch culture.
Therefore, stable co-existance of two organisms can be regarded as the feature of an ideal biofuel producer in the future.6
Broad Spectrum Substrate Range
Coutilization of hexoses and pentoses derived from lignocellulose is an attractive trait in microorganisms considered for consolidated biomass processing to biofuels. Hexoses and pentose sugars degradation by microbes can be considered for biofuel processing.7 In the process of biofuel production, the tough polysaccharides are deconstructed in to simple sugars by various techniques.8 Efficient degradation will definitely lead to attractive fuels.9 For this reason, there is a great desire in identifying the microbes that are efficient in carbohydrate degradation in to simple sugars.10 In the environment, microbial survival can be based on the capacity to utilize a variety of available carbohydrates as carbon and energy sources.11 The issue was examined for the extremely thermophilic bacterium C.saccharolyticus growing re is a great desire in identifying the microbes that are efficient in carbohydrate degradation in to simple sugars.10 on individual monosaccharides (arabinose, fructose, galactose, glucose, mannose & xylose) and mixture of these sugars. The organism grew at approximately the same rate and to the same final density on all sugars ans sugar mixtures tested.12 C.saccharolyticus monosaccharide co-fermentation shows the xylose usage is much greater than glucose 13 Its capacity to coutilize other sugars and the extent of utilization of indivir biofuel productiondual sugars present in the mixture of pentoses and hexoses is analysed (Fig. 2) .Therefore, C.saccharolyticus can metabolize a number of monosaccharides simultaneously without exhibiting carbon catabolite repression.
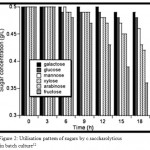 |
Figure 2: Utilisation pattern of sugars by c.saccharolyticus in batch culture12
|
Special Domain Proteins
Members of extremely thermophilic genus ‘caldicellulosiruptor’ have potential as consolidated bioprocessing (CBP) microorganisms because of their capacity to convert plant-based polysaccharides directly in to a biofuel in a growth associated manner 14 Caldicellulosiruptor saccharolyticus has 11S-layer homology (SLH) domain proteins, presumably to enable binding to the S-layer. SLH domains 50 to 60 aminoacids (aa) long have been identified at the amino-terminal region of S-layer proteins and at the carboxy-terminal end of cell associated extracellular enzymes.15 S-layer motifs specially recognize pyruvylated secondary cell wall polymers (SWCPS) as the anchoring structure.16 Two SLH domain containing proteins Csac_0678 and Csac_2722 are present. The Csac_0678 gene encodes a glycosidase hydrolase (GH) family 5 catalytc domain.17 This protein associates with the S-layer and may play a specific role in utilization of insoluble substrates by C.saccharolyticus.
Csac_2722 represents the largest ORF in the Caldicellulosiruptor saccharolyticus genome, comprised of 2,593 aminoacids. It has no discernible catalytic domains but does contain two big 4 domains arranged in tandom with two galactose-binding domain-like domains (GDB), one adherin –like domain and three SLH domains. Cadherin like domains appeared to contribute to protein-protein interactions and carbohydrate binding.18 The two proteins have the capacity and contribute much to the deconstruction of plant biomass (Fig3).
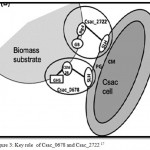 |
Figure 3: Key role of Csac_0678 and Csac_2722 17
|
Unique Adaptability to A Variety of Environmental Conditions
C.saccharolyticus was isolated from a piece of wood in a hot-spring in Rotorua-Taupo thermal area in New Zealand, of which the waters have neutral PH and are low in sulphate, salt content and free sugars,20with majority of being glucose and xylose contained in complex (hemi) cellulose polymers, which are difficult to access. C.saccharolyricus is well adopted to the selective pressure of this habitat through the production of various hydrolyzing enzymes, possession of a wide range of high-affinity transport systems and absence of glucose-based catabolite repression. Genomic analysis throws light over the high number of transposons and transposable derivatives the C.saccharolyticus possess as compared with other organism.21 Since transposition increases the chance of genetic alterations, enhancing the adaptability of this organism.
‘Tapirins’ Mediating Cellulose Adherence
It has been established for some time that non-catalytic, biomolecular contributions are critical to the degradation of crystalline cellulose22.Bioinformatics and Proteomic analysis identified structurally unique, discrete, hypothetical proteins called ‘tapirins’ (Origin from Maori : to join), not directly associated with cellulases, that mediate attachment to cellulose by species in the non-cellulosomal, extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus.23
Two tapirin genes are located directly downstream of a type IV pilus operon in strongly cellulolytic members of genus caldicellulosiruptor.. Based on aminoacid sequence homology, the tapirins are unique proteins currently only found in the genus caldicellulosiruptor.23 Overall, the tapirins significantly establish a new paradigm by which lignocellulose degrading microbes can attach to plant biomass.
Dominance of Thermophiles Among Extremophiles in Biofuel Production
Extremophiles refer to organisms mostly prokaryotic, which thrive in environmental conditions considered to be hostile to humans. The parameters which contribute towards the extreme conditions include temperature, PH, salinity, pressure, radiation, light, and water content.
Many steps in biofuel production involve high temperatures and extremes of PH, therefore extremophiles are ideal candidates to replace the mesophilic organisms used in traditional methods. The current market for biofuels rely on the use of substrates like lignocellulosic biomass whose conversion to biofuel rely through microbial catalysis. Extremophiles are a robust group of organisms producing stable enzymes, which are often capable of tolerating changes in the environmental conditions. The potential application of such organisms and their enzymes in biotechnology is enormous as it is more cost-effective and has gained great momentum over the last several years.
The use of extremophiles in biofuel production has become apparent that the majority are of ‘thermophilic’ source. To date, thermophiles like caldicellulosiruptor saccharolyticus have found their way in to large scale in the field of biotechnology in terms of biofuel production. This is not surprising since thermophiles have a remarkable ability to tolerate fluctuations in PH, temperature and environmental changes,24 an attribute which offers a clear advantage In the development of a commercially viable process.25
Thermophiles readily ferment pentose and hexose sugars from biomass and in some cases even structurally complex carbohydrates, a quality which is particularly important for production of second generation biofuels.26 (Table 2)
Table 2: List of Thermophilic bacteria and their substrates27
| Thermophilic Bacteria | Location | T opt (C) | Source | Biomass substrates |
| T.maritima | Vulcano island. Italy | 80 | Marine sediments | alpha, beta, H, P |
| T.lettingae | Netherlands | 65 | Sulfate-reducing bioreactor | alpha, beta, H,P |
| T.petrophila | Nigata, Japan | 80 | Kulbiki oil reservoir | alpha |
| Ca.saccharolyticus | Taupo, New zealand | 70 | Wood from hot spring | alpha, beta, C, H, P |
| C.thermocllum | Louisiana, USA | 60 | Cotton bale | C |
| Thermotoga sp. RQ2 | Ribeira Quente, the Azores | 76-82 | Marine sediments | alpha |
Biomass Substrates Are Abbreviated As Follows : Alpha : Alpha-Linked Glucans, Beta : Beta-Linked Glucans; C : Crystalline Cellulose; T: Chitin; H: Hemicelluloses; P: Pectin
Furthermore thermophilic industrial applications are less prone to microbial contamination and require lower energy inputs as a result of the reduced cooling steps needed between the fermentation steps with the removal of any volatile products, which in turn minimizes the problem of product inhibition. Hence a lot of dominance of thermophiles.
Conclusion
Economically, biofuels will not be able to replace the demand for fossil fuels unless lignocellulosic biomass and waste water are used in the fermentation process. Extremophiles have an extensive food hold in the market, that is expected to keep growing. However, to fulfill this great potential, innovative methods will have to be developed to overcome current road blocks. The insights in to the biological chemistry of caldicellulosiruptor saccharolyticus to a better way of utilizing the organism in finding the substitutes for fossil fuels, as this organism bears all the hallmarks of being adopted as a ‘biofuel producer’. However, for obvious economical reasons, the performance of the organisms needs to be improved, as the day is not too far when microbes could well be the key to powering cars in an environmentally sound way and in the not too distant future we would all be filling up at the pump with microbial-based fuel
Acknowledgement
I, G. Sai Vaishnavi, extremely thankful to the funding source Ministry of Science & Technology & Department of Science & Technology (DST), Govt of India for providing INSPIRE Fellowship (No. DST/INSPIRE Fellowship/2014/IF140723) & Sri Krishnadevaraya University, Ananthapuram, Andhrapradesh for the concern and support in the research work. We do not have any conflict of interest.
References
- Nizami A. S., Ismail I. M. L. Lifecycle assessment of biomethane from lignocelluloses biomass in : Life cycle assessment of renewable energy sources. Green Energy and Technology book series, Springer-Verlag, London. 2013
- Ouda O. K. M. Raza S. A., Nizami A. S.,Rahan M. Al-Waked., R.korres N. E. Waste to energy potential: A case study of Saudi Arabia. Renew. sust. Energy. rev. 2016;61:328-340.
- Singh A.,Pant D.,Olsen S. I., Nigam P. S. Key issues to consider in icroalgae based biodiesel production. Energy Educ. Sci. Technol. Part A. Energy Sci Res. 2012;29(1):563-576.
- Sara E. Blumer-Schuette., Derrick L. L., Robert M. K. Phylogenetic microbiological and glycoside hydrolase diversities with in the extremely thermophilic, plant biomass-degrading genus caldicellulosiruptor. Appl.Environ.Microbiol. 2010;76(24):8084-8092.
CrossRef - Stolyer S. V., Dien S., Hillerland K. L, Pinel N., Lie T. J., Stahl D. A. Metabolic modeling of a mutualistic microbial community. Mol. Syst. Biol. 2007;3:92-10.1038/msb4100131.
- Ahmad A. z., Red P. S., Ed W. J.,Nicl V. Stable co-existance of two Caldicellulosiruptor saccharolyticus in a denovo constructed hydrogen producing co-culture. Microbial cell Factories. 2010;9(102):10.1186/1475-2859-9-102.
- Liu C. F., Ren J. L., Xu F., Liu J. J., Sun J. X., Sun R. c. Isolation and characterization of cellulose obtained from ultrasonic irradiated sugarcane baggase. J. Agric. Food chem. 2006;54:5742-5748.
CrossRef - Sanchez O. J., cardona. Trends in biotechnological production of fuel ethanol from different feed stocks. Bioresource Technol. 2008;99:5270-5295.
CrossRef - Lynd l. R. Overview and evaluation of fuel ethanol from cellulose biomass: Technology economics the environment and policy. Annu. Rev. Energy Environ. 1996;21(403-465).
CrossRef - Kim J. H.,Shoemaker S. P., Mills D. A. Relaxed control of sugar utilization in Lactobacillus brevis. Microbiol. 2009;155:1351-1359.
CrossRef - Kovarova-Kovar K., Egli T. growth kinetics of suspended microbial cells from single-substrate controlled growth to mixed- substrate kinetics. Microbiol. Mol. Biol. Rev. 1998;62:646-666.
- Amy L.V. F., Marcel R. A. v., Serve M. W. K., Kelly R. Carbohydrate utilization patterns for the extremely thermophilic bacterium caldicellulosiruptor saccharolyticus reveal broad growth substrate preferences. Appl. Environ. Microbiol. 2009;75(24):7718-7724.
CrossRef - De Z. T. V., Budde M. a., Szengyel Z., Rec K. Z and Classen P. A. Hydrogen production from paper sludge hydrolysate. Appl.Biochem. Biotechnol. 2003;105(108):557-566.
- Herbel Z.,et.al. Exploitation of the extremely thermophilic Caldicellulosiruptor saccharolyticus in hydrogen and biomass production from biomasses. Environ. Technol. 2010;31:1017-1024.
CrossRef - Ries W., Hotzy C., Schocher I., sletyr U. B., Sara M. Evidence that the N-terminal part of the S-layer protein from Bacillus stearothermophilus Pv 72/P2 recognizes a secondary cell wall polymer. J. Bacteriol. 1997;179:3892-3898.
CrossRef - Ferner-Ortner-Bleckmann J., et.al. The high molecular mass amylase (HMMA) of Geobacollus stearothermophilus ATCC 12980 interacts with the cell components by virtue of three specific binding regions. Mol.Microbiol. 2009;72:1448-1461.
CrossRef - Cantarel B. L., et.al,. The carbohydrate-Active enzymes database (CAZy) An expert resources for glycogenesis. Nucleic Acids Res. 2009;37:233-238.
CrossRef - Fraiberg M., Borovock I., weiner R. M., Lamed R. Discovery and characterization of Cadherin domains in Saccharophagus degradans 2-40. J. Bactriol. 2010;192:1066-1074.
CrossRef - Boraston A. B., Bolam D. N., Gillbert H. J., Davies G. J. Carbohydrate-binding modules: Fine tuning polysaccharide recognition. Biochem.J. 2004;382:769-781.
CrossRef - Sissons C. H., Sharrock K. R., Daniel R. M., Morgan H. W. Isolation of cellulolytic anaerobic thermophiles from New Zealand thermal sites. Appl .Environ Microbiol. 1987;53:832-838.
- chowdary N., selvaraj A., Krishna l. k., ramesh G. K. Genome wide re-annotation of caldicellulosiruptor saccharolyticus with new insights in to genes involved in biomass degradation and hydrogen production. Journal Pone. 2015;10:1371.
- Sara E. Blumer-Scheutte., Alahuhta M., Jonathan M. C., Loura L. L., Jeffery V. Z., Rishard J. G., Robert M. K. Discrete and structurally unique proteins (tapirins) mediate attachment of extremely thermophilic Caldicellulosiruptor species to cellulose. J of Biol.chem. 2015;290(17).
- Glansdorff C. N. Physiology and biochemistry of extremophiles, Washington, DC : ASM Press. 2007.
- Dien B. S., Cotta M. A., Jeffries T. W. Bacteria engineered for fuel ethanol production: Current status. Appt.Microbiol.Biotechnol. 2003;63:258-266.
CrossRef - Georgieva P. T., Ahring B. K. Potential for using tharmophilic anaerobic bacteria for bioethanol production from hemicellulose. Biochem. Soc. Trans. 2004;32:283-289.
CrossRef - Sara E. Blumer-schuette., kataeva I., westpheling J., Michael W. W A., Robert M K. Extremely thermophilic microorganisms for biomass conversion: status and Prospects. current opinion in biotechnology. 2008;19:210-217.
- Pande M., Ashok N. B. Biomass Conversion to energy. Journal of Biomass Conversion. 2012;1-90
- Rathore D., Nizami A. S., Singh A., Pant D. Key issues in estimating energy and green house gas savings of biofuels: Challenges and Perspectives. Biofuel Research Journal. 2016;3(3):380-393.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





