How to Cite | Publication History | PlumX Article Matrix
P. Rajeswari1 and Rupam Kapoor2
1UGC Post- Doctoral Women Fellowship, Department of Botany, University of Delhi, India.
2Department of Botany, University of Delhi,110007 India.
Corresponding Author E-mail: aksharaasmitha @gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2557
ABSTRACT: Fusarium oxysporum causes Fusarium wilt of crop plants leads to considerable yield loss. The study was conducted to determine the beneficial effects of combining Trichoderma species and Pseudomonas fluorescens i.e Trichodema viride+ Pseudomonas fluorescens (Tv+Pf) (1+2%), Trichoderma harzianum+Pseudomonas fluorescens (Th+Pf) (1.5+2%), Trichoderma viride +Trichoderma harzianum (Tv+Th) (1+1.5%) on the activity of cellulolytic enzymes of Fusarium oxysporum to control Fusarium wilt of Arachis hypogaea. L wilt in vitro. The activity of 1,4 -β – Endoglucanase, 1,4 -β – Exoglucanase, Cellobiases produced by Fusarium oxysporum (Control) was higher. Maximum inhibition of Cellulolytic enzymes was shown by culture filtrate of Trichoderma viride + Pseudomonas fluorescens (Tv+Pf) (1+2%), followed by Trichoderma harzianum + Pseudomonas fluorescens, (Th +Pf) (1.5+2%) and Trichoderma viride + Trichoderma harzianum (Tv+Th) (1+1.5%). However, disease suppression of Fusarium wilt of Arachis hypogaea. L by the compatible combination of Trichodema viride + Pseudomonas fluorescens (1+2%) was considerably better as compared to other two strains. At the same time the other two combinations resulted in enhanced disease suppression as compared to single strains. This indicates that the potential benefits of using combination treatments to suppress Fusarium wilt. The study suggests the significance of interactive effects of Trichoderma and Pseudomonas in biocontrol of wilt disease.
KEYWORDS: Arachis hypogaea. Fusarium wilt; In vitro interactions; Trichoderma viride; Trichoderma harzianum; Pseudomonas fluorescens; L
Download this article as:| Copy the following to cite this article: Rajeswari P, Kapoor R. Combined Application of Different Species of Trichoderma and Pseudomonas Fluorescens on the Cellulolytic Enzymes of Fusarium Oxysporum for the Control of Fusarium Wilt Diseasei Arachis Hypogaea. L. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Rajeswari P, Kapoor R. Combined Application of Different Species of Trichoderma and Pseudomonas Fluorescens on the Cellulolytic Enzymes of Fusarium Oxysporum for the Control of Fusarium Wilt Diseasei Arachis Hypogaea. L. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27443 |
Introduction
Fusarium oxysporum was considered as the most important phytopathogenic and toxigenic genus of filamentous fungi worldwide (O’Donnell, 1996; Langseth et al., 1999; Eskola et al., 2001; Kosiak et al., 2003; Zhang et al., 2007; Suga et al., 2008). Fusarium wilt caused by Fusarium oxysporum (Schlecht. Emend.Snyder& Hansen) leads to significant loss to crop yield and quality of nuts in groundnut plants. Cellulose is the main component of the plant cell wall. It is synthesized by plasma membrane protein complexes. It is deposited directly on the wall in a directional way (Taylor N.G 2008). It is a linear homopolymer composed of β (1→4) linked d-glucose units. Cellobiose is a glucose dimer linked by β (1→4) and found as repeating unit of cellulose. It is found that plant pathogens produce the exocellular enzymes like cellulolytic, hemi cellulolytic, pectinolytic and proteolytic enzymes which degrade the major components of Cellwall (Wheeler, 1975; Riou,1991). Most pathogens capable of producing more cellulolytic than pectolytic enzymes (Sadik et al., 1983). The chemistry of cellwall affects by enzymes produced by pathogens and further leads to cellwall degradationI (Albersheim and Jones 1969; Miettinen- Oinnonen and Suominen 2002). The production of cellulolytic enzymes and degradation of cellulose by several fungi has been reported by many workers, (Basu and Ghose 1960; Bateman 1964; Sohaila et al., 2011). The studies reported that most of the plant pathogenic organisms are capable of degrading cellulose by producing a cellulose complex which involves the synergistic action of three main enzymatic complexes.1 endo-β1-4-glucanases (endo-β1-4-d-glucan 4-glucanohydrolase,2 exo-β1-4-glucanase or cellobiohydrolase (exo- β1-4-d-glucan 4-cellobiohydrolase, and3 β-glucosidase or cellobiase. These components act synergistically to hydrolyze cellulose to glucose. Endo-glucanase hydrolyzes internal β-1,4 linkages of cellulose chains and creates new reducing and non-reducing ends. Exoglucanase cleaves disaccharide cellobiose from the non-reducing end (cellobiohydrolase). In some cases, it cleaves from the reducing end (cellobiosidase) of the cellulose chain. These cellobiose units and short-chain cello dextrins are hydrolyzed by β-glycosidase into individual monomeric units of glucose (Nelson 1994; Moreria 2005; Beguin and Aubert 1994). Cellulases is associated with pathogenicity of number of microorganisms (Jan and Chen 2003). There are many studies on biological control of soil borne diseases caused especially by Fusarium oxysporum (Marois et al., 1981; Sivan and Chet, 1986). It was successfully demonstrated that biological control of Fusarium wilt by Trichoderma spp and Pseudomonas spp Saravanan et al., 2003; Tu and Chang 1983; Duijff et al., 1999). Combined application of different biocontrol agents has improved disease protection (Jetiyanon and Kloepper 2002). Studies on banana demonstrated that biocontrol agents in different combinations have improved plant growth parameters than individual treatments by reducing the Fusarium oxysporum f.sp. cubense infection in field conditions. (Sukhada et al., 2010). Abeysinghe (2009) suggested that combination of B. subtilis with Pseudomondas strains can lead to greater plant protection against R. solani and S. rolfsii than individual application. Combining antagonists plays more effective biological control treatments of infection rather than individual antagonists (Janiesiewicz 1996). Weller (1994) found that combinations of several fluorescent Pseudomonads have greater biocontrol activity against take all of wheat as compared to application of single strains. Wahid (2006) reported that combination of biocontrol agents gave better results than using them singly. Talaviya and Jadega (2015) established that combined application of T.viride+T.harzianum+ Pseudomonas fluorescens was found most effective in controlling cumin wilt disease and also had highest seed yield. There seems to be no report on the control of Fusarium wilt with the combinations of biocontrol agents on Arachis hypogaea.L. The present study was taken to determine the significant interactive effects of Trichoderma spp and Pseudomonas fluorescens in biocontrol of Fusarium wilt of Arachis hypogaea L. in comparison with application of single strains.
Materials and Methods
Microbial Cultures
The bacterial and fungal cultures used in the study were obtained from Institute of Microbial Technology (IMTECH), Chandigarh. Fusarium oxysporum was cultured on Potato Sucrose Agar(PSA) for 30 days. Trichoderma viride and Trichoderma harzianum were cultured on Malt Extract Agar(MA) and Pseudomonas fluorescens on Antartic biotic Medium(ABM) for 30 days. All these cultures further grown on Czapek’s medium separately for 7 days at 28°±0.2C. The culture filtrates were taken after centrifuged.
Enzyme Production
For the assay of 1,4 β – endoglucanases, Czapek’s broth was supplemented with carboxy methyl Cellulose and for 1,4- β exo glucanases microcrystalline cellulose was used. Culture filtrates of Tv+Pf (1+2%), Th+Pf (1.5+2%) Tv+Th (1+1.5%) Tv+Th (1+1.5 %) in their OIC (Optimum Inhibitory Concentration) were amended to 50ml Czapek’s liquid media separately. The two discs of 9 mm of F. oxysporum was inoculated in each flask and incubated in the BOD incubator at 28°± 0.2C for 7 days. The control (with Fusarium oxysporum) and treated flasks (Tv+Pf, Th+Pf, Tv+Th) were all maintained in triplicates. After incubation, the fungal mat and the liquid media were separated by Whatman No.1 filter paper. The filtrates were further centrifuged at 5000 rpm for 10 min and the supernatant was used as the enzyme source.
Assay of 1,4 -β – Endoglucanase (Cx) Activity
The activity of 1,4 endoglucanases was assayed as per the method described by Mahadevan and Sridhar (1986) and the enzyme activity was calculated as percent reduction in viscosity of the substrate. The reducing sugars released by the enzyme sources in the same reaction mixture was estimated according to Wang et al., (1997).
Substrate Preparation
Carboxyl Methyl Cellulose (0.5g) was dissolved in 100 ml of sodium acetate-acetic acid buffer with pH 5.2 and kept in water bath at 50-60°C for 5-10 min. The substrate was filtered and stored at 4°C with a layer of toluene.
Ostwald viscometer 150 size was used to determine the viscosity loss of cellulose substrate.
4 ml of carboxyl methyl cellulose, 1 ml of the buffer and 2 ml of enzyme was pipetted out into the viscometer. The contents were mixed well and the efflux time of the mixture was determined at every 30-min interval for 3 h incubation and the enzyme activity was calculated from the following formula.
T0-T1
V= ______× 100
T0-TW
Where V = percent loss in viscosity To = flow time of reaction mixture at 0 minute
T1= flow time of reaction mixture at a particular time interval Tw = flow time of distilled water
Assay of 1,4 -β – Exoglucanase activity
1,4- β-Exo glucanase activity was measured by estimating the reducing sugars released by the breakdown of avicel with anthrone reagent. To 1 ml of enzyme source, 1 ml of buffer and 0.5 ml of substrate were added in a test tube and incubated at room temperature for 2 h. The reaction mixture was mixed well and centrifuged. To 2 ml of the above supernatant, 3 ml of orcinol reagent was taken in the test tubes and 10 ml of anthrone reagent was added on ice. The tubes were mixed well and heated in a water bath at 80°C exactly for 20 minutes and immediately cooled under running tap water. The colour developed was read at 485 nm in Systronics Spectrophotometer. A blank was prepared with 2% H2S04 instead of orcinol. Control was maintained with boiled enzyme reaction mixture and with zero-time reaction mixture.
Estimation of Cellobiase
To estimate the cellobiase enzyme, the mixture of 1.5 ml of the buffer, 2.5 ml of 5 mM cellobiose and 1 ml of the enzyme was incubated at 30°C for 2 h. Then the reaction was terminated. By using DNS (dinitro salicyclic acid) reagent, the amount of glucose liberated by the enzyme was measured at 575 nm in Systronic Spectrophotometer. Glucose was used as standard.
Statistical Analysis
The data of experiment was statistically analyzed according to ANOVA and significance with in the column with Tukey HSD multiple range test (TMRT) at 5% level of significance (n=3)
Results and Discussion
The 1,4 -β – Endoglucanase of control reduced the viscosity of the substrate to 80% at 180min. The lowest activity was observed in the enzyme source obtained from the culture treated with Trichoderma viride + Pseudomonas fluorescens (Tv+Pf) (13.3% viscosity loss at 180 min) followed by Trichoderma harzianum + Pseudomonas fluorescens (Th+Pf) (17.39%) and Trichoderma viride + Trichoderma harzianum(Tv+Th) (21.05%). (Fig.1)
The activity of 1,4 -β – Exoglucanase was expressed in specific activity units (SAU). All the treatments inhibited the activity of 1,4 -β –Exo-glucanase at varying degree. Higher amount of monogalacturonic units was released in the case of enzyme source obtained from the control (487.53 SAU), followed by Tv+Th (Trichoderma viride+ Trichoderma harzianum) (234.00 SAU) and Th+Pf (Trichoderma harzianum + Pseudomonas fluorescens) (165.05 SAU). Among the treatments the least amount of sugar was liberated in the case of enzyme source obtained from treatment Tv+Pf (Trichoderma viride + Pseudomonas fluorescens) (95.14 SAU). (Table 1).
The Cellobiase activity was observed in enzyme source of control (297.36 SAU). The lowest rate of enzyme activity was observed in Tv+Pf (Trichoderma viride + Pseudomonas fluorescens)-treated enzyme source (91.50 SAU) followed by Trichoderma harzianum + Pseudomonas fluorescens Th+ Pf (155.12 SAU) and Trichoderma viride + Trichoderma harzianum and (Tv+Th) (195.18 SAU). (Fig.2)
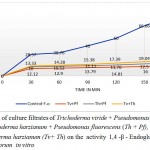 |
Figure 1: Effect of culture filtrates of Trichoderma viride + Pseudomonas fluorescens (Tv + Pf), Trichoderma harzianum + Pseudomonas fluorescens (Th + Pf), Trichoderma viride+ Trichoderma harzianum (Tv+ Th) on the activity 1,4 -β – Endoglucanases of Fusarium oxysporum in vitro
|
Table 1: Effect of culture filtrates of Trichoderma viride + Pseudomonas fluorescens (Tv + Pf), Trichoderma harzianum + Pseudomonas fluorescens (Th + Pf), Trichoderma viride+ Trichoderma harzianum (Tv+ Th) on the activity 1,4 -β – Exoglucanases of Fusarium oxysporum in vitro
| In Vitro | SAU(Specific Activity Units) |
| Control-Fusarium oxysporum | 487.53±0.30 |
| Treatment with Tv+Pf(Trichoderma viride + Pseudomonas fluorescens) | 95.14±0.52 a |
| Treatment with Th+Pf(Trichoderma harzianum + Pseudomonas fluorescens) | 165.05±0.95 a |
| Treatment with Tv+Th(Trichoderma viride+ Trichoderma harzianum) | 234±0.44 a |
ap< 0.001 as compared to control SAU= micro g of maltose equivalent liberated /h
The values within a column followed by different letters are significantly different according to Tukey’s HSD multiple range test (TMRT) at 5% level of significance(n=3)
The Cellobiase activity was observed in enzyme source of control (297.36 SAU). The lowest rate of enzyme activity was observed in Tv+Pf (Trichoderma viride + Pseudomonas fluorescens)-treated enzyme source (91.50 SAU) followed by those treated with Trichoderma harzianum + Pseudomonas fluorescens Th+ Pf (155.12 SAU) and Trichoderma viride + Trichoderma harzianum and (Tv+Th) (195.18 SAU).(Fig.2)
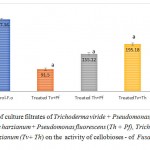 |
Figure 2: Effect of culture filtrates of Trichoderma viride + Pseudomonas fluorescens (Tv + Pf), Trichoderma harzianum + Pseudomonas fluorescens (Th + Pf), Trichoderma viride + Trichoderma harzianum (Tv+ Th) on the activity of cellobioses – of Fusarium oxysporum in vitro
|
ap< 0.001 as compared to control SAU= micro g of maltose equivalent liberated /h
The values within a column followed by different letters are significantly different according to Tukey’s HSD multiple range test (TMRT) at 5% level of significance(n=3)
Maximum inhibiton of 1,4 β Endoglucanases, 1,4 Exoglucanases, and Cellobiase enzyme activity was recorded in Tv+Pf (Trichoderma viride + Pseudomonas fluorescens) treated culture. Our results are in agreement with the earlier studies recorded by Karthikeyan et al. (2006) that T. viride, P. fluorescens, neem cake, T. viride + neem cake, P. fluorescens + neem cake that applied to soil has considerably reduced the stem rot incidence compared to control in groundnut plants 60 days after sowing. Khan et al. (2004) reported that combination of Trichoderma and Pseudomonas has better efficacies in control of Fusarium infection on chickpea than using them singly.
Salman et al. (2017) concluded that application of Pseudomonas to field crops such as watermelon and similar crops that are affected by Fusarium spp improve their productivity and yields of such crops. The mixtures of bioagents also acts a PGPR. The phytohormones viz. auxins, gibberellins, cytokinins and ethylene produced by bacteria play a major role in growth promotion. The same is reported by Lehar et al. (2016) stating that administration of biological agents of T. viride combined with P. fluorescens and Streptomyces sp. produce growth hormone or PGPR which stimulates better plant growth and thereby increasing yield in potato and ability to control disease caused by Phytophthora infenstan and Ralstonia solanacearu.
Our results indicated that the inhibition of 1,4 β Exoglucanases, 1,4 β Endoglucanases, and Cellobiases was recorded in Th+Pf (Trichoderma harzianum + Pseudomonas fluorescens) treated culture. Our results substantiate earlier reports that in management of plant diseases the use of biocontrol agents in combinations were more effective than individual agents (Thilagavathi et al., 2007; Ganeshmoorthi et al., 2008). The application of T.harzianum, captan and neem seed extract two days after pathogen inoculation significantly reduced damping off disease caused by S. rolfsii in greenhouse grown tomato plants (Okereke and Wokocha 2006). Thangavelu and Gopi (2015) found that the combination of bacterial antagonists could provide sustainable management of Fusarium wilt of banana under field conditions.
Our results also revealed that inhibition of 1,4 Exoglucanases 1,4 β Endoglucanases and Cellobiases was recorded in Tv+ Th (Trichoderma viride + Trichoderma harzianum) treated culture. Similar observation were recorded by Rini and Sulochana (2007) that T.viride and T. harzianum, with P . fluorescens were found to be compatible and more effective in suppressing the seedling disease of chilli and tomato.
Bosah et al. (2010) reported that Trichoderma spp. proved to be the most effective biocontrol agent against S. rolfsii in inhibiting the growth of the pathogen by 80% under in vitro conditions. In greenhouse trial, inoculation of mint plants with either T. harzianum or T. virens significantly re-duced the collar rot caused by S. rolfsii and was accompanied by significant increase in herb and oil yield (Singh and Singh 2004).
Conclusion
Results from the present study revealed that cellulolytic enzymes (Exo and endo Glucanases, and Cellobiases) produced by Fusarium oxysporum were inhibited by culture filtrate of Trichoderma viride+Pseudomonas fluorescens (1+2%) followed by Trichoderma harzianum+ Pseudomonas fluorescens (1.5+2%) and Trichoderma viride +Trichoderma harzianum(1+1.5%). The different disease suppression mechanisms together lead to enhanced disease suppression by the combined application of above strains. The reasons for the reduced wilt incidence and severity may attributed to various mechanisms of Trichoderma spp. viz. mycoparasitism, spatial and nutrient competition, antibiosis by enzymes and secondary metabolites, and induction of plant defence system. The Pseudomonas produced secondary metionabolites like phenazine, 2,4-diacetylphloroglucinol, pyocyanine, pyoluteorin and pyrrolnitrin which were involved in enhancing plant growth by suppressing the disease caused by Fusarium oxysporum. In this study combinations of biocontrol agents showed significant reduction in Fusarium wilt disease. The application of fungal and bacterial combination, i.e. T. viride + P. fluorescens (1+2%) has proved to be more effective in managing the Fusarium wilt in Arachis hypogaea L as compared to other combinations and single strains.
Acknowledgements
We are thankful to UGC University Grant Commission (India) for funding and to pursue the research under the category of Post-Doctoral Fellowship for Women.
Conflict of Interest
The co-author Prof. Rupam Kapoor is the mentor/ Research supervisor for my research project. The authors have no conflict of interest. On behalf of both authors, I am endorsing the same.
Funding Source
The study was funded by UGC(India). The corresponding author Dr. P. Rajeswari has received research grant for Post-doctoral research project titled Biochemical effects of combinations of Trichoderma spp and Pseudomonas fluorescens against Fusarium wilt of Arachis hypogaea.L(Groundnut)under the category of Post-Doctoral Fellowships for Women(F.15-1/2015-17/PDFWM-2015-17-AND-34147(SA-II)
References
- Abeysinghe S. Efficacy of combined use of biocontrol agents on control of Sclerotium rolfsii and Rhizoctonia solani of Capsicum annuum. Archives of Phytopathology and Plant Protect. 2009;42:221-227.
CrossRef - Albersheim P., Jones T. M., English P. D. Biochemistry of the cell wall in relation to the infective processes. Annu. Rev. Phytopathol. 1969;7:171–194.
CrossRef - Basu S. N., Ghosh S. N. The production of cellulose by fungi on mixed cellulosic substances. Can. J. Microbiol. 1960;6:265-288.
CrossRef - Bateman D F. Cellulase and Rhizoctonia disease of bean. Phytopathol. 1964;54(11):1372-1377.
- Beguin P., Aubert J. P. The biological degradation of cellulose. FEMS Microbiol Rev. 1994;13:25–58.
CrossRef - Bosah O., Igeleke C. A., Omorusi V. I. In vitro microbial control of patho- genic Sclerotium rolfsii. International Journal of Agriculture and Biol. 2010;12:474-476.
- Duijff B. J., Recorbet G., Peter A., Bakker H. M., Loper J. E & Lemanceau P. Microbial antagonism at the root level is involved in the suppression of Fusarium wilt by the combination of nonpathogenic Fusarium oxysporum Fo47 and Pseudomonas putida WCS358. Phytopathol. 1999;89:1073-1079.
CrossRef - Eskola M., Parikka P., Rizzo A. Trichothecenes, ochra- toxin A and zearalenone contamination and Fusarium in- fection in Finnish cereal samples in 1998. Food Additives and Contamin. 2001;18:707-718.
CrossRef - Ganeshamoorthi P., Anand T., Prakasam V., Bharani M., Ragupathi N., Samiyappan R. Plant growth promoting rhizobacterial (PGPR) bioconsortia mediates induction of defense-related proteins against infection of root rot pathogen in mulberry plants. Journal of Plant Interac. 2008;3(4):233-244.
CrossRef - Jan H. D., Chen K. S. Production and characterization of thermostable cellulases from Streptomyces transformant T 3-1. World J of MicroBiol.Biotec. 2003;19:263-268.
- Janisiewiez J. Ecological diversity, niche overlap and coexistence of antagonists used in developing mixtures for biocontrol of post-harvest diseases of apples. Phytopathol. 1996;86:473-479.
CrossRef - Jetiyanon K., Kloepper J. W. Mixtures of plant growth promoting rhizo- bacteria for induction of systemic resistance against multiple plant diseases. Biological Cont. 2002;24:285-291.
CrossRef - Karthikeyan V., Sankaralingam A., Nakkeeran S. Biological control of groundnut stem rot caused by Sclerotium rolfsii (Sacc.). Archives of Phyto- pathol and Plant Protect. 2006;39:239-246.
CrossRef - Khan M. R., Khan S. M., Mohiddin F. A. Biological control of Fusarium wilt of chickpea through seed treatment with the commercial formulation of Trichoderma harzianum and Pseudomonas fluorescens. Phytopathologia Mediter. 2004;43:20–25.
- Kosiak B., Torp M., Skjerve E. The prevalence and dis- tribution of Fusarium species in Norwegian cereals a survey. Acta Agriculturae Scandinavica Section B- Soil and Plant Sci. 2003;53:168-176.
- Langseth, W., Bernhoft, A., Rundberget, T., Kosiak, B., Gareis, M. Mycotoxin production and cytotoxicity of Fusari- um strains isolated from Norwegian cereals. Mycopathol. 1999; 144: 103-113.
CrossRef - Laurensius L.,Tatik W., Maghgoer M. D.Selection of Potato varieties. (S. tuberosum.L) in midlands and the effect of using biological agents. Int. Jour.of Biosci. 2016;9:129-138.
- Mahadevan A., Sridhar R. Methods in physiological plant pathology (3rd ed.)1986; Sivakami Publications, Madras, India.
- Marois J. J., Mitchel D. J., Somada R. M. Biological control of Fusarium crown and root rot of tomato under field condition. Phytopathol. 1981;12:1257-1260.
- MiettinenOinnonen A., Suominen P. Enhanced production of Trichoderma reesei endoglucanses and use of the new cellulase preparations in producing the stonewashed effect on denim fabrics. Appl. Environ. Microbiol. 2002;68:3956–3964.
CrossRef - Moreira F. G., Simone R., Costa M. A. F., de Souza C. G. M., Peralta R. M. Production of hydrolytic enzymes by the plant pathogenic fungus Myrothecium verrucariain submerged cultures. Braz.J. Microbiol. 2005;
36:7-11.
CrossRef - Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1994;153:375-80.
- O’Donnell K. Progress towards a phylogenetic classification of Fusarium. Sydowi. 1996;48:57-70.
- Okereke V.C. Wokocha R. C. Effect of some tropical plant extracts, Trichoderma harzianum and captan on the damping-off disease of tomato induced by Sclerotium rolfsii. Agric. J. 2006;1:52-54.
- Pierson E. A., Weller D. M. Use of mixtures of fluorescent pseudomonas to suppress take –all and improve the growth of wheat Phytopathol. 1994;84:940-947.
CrossRef - Rini C. R., Sulochana K. K. Usefulness of Trichoderma and Pseudomonas against Rhizoctonia solani and Fusarium oxysporum infecting tomato. Journal of Tropical Agricult. 2007;45:21-28.
- Riou C., Freyssinet G., Fevre M. Production of cell wall degrading enzymes by the Phytopathogenic fungus Sclerotinia sclerotiorum. Appl. Environ. Microbiol. 1991;57(5):1478-1484.
- Sadik E. A., Payak M. M., Mehta S. L. Some biochemical aspects of host-pathogen interactions in Pythium stalk rot of maize. I. Role of toxin, pectolytic and cellulolytic enzymes in pathogenesis. Acta Phytopathol. Acad. Sci. Hungaricae. 1983;18:261-269.
- Salman M.,Shahin N.,NawafAbu-Khala N.,Jawabrih M.,Rumaileh B. A.,Abuamsh R. R., Sameer A., Sameer A. Antagonistic activity of Pseudomonas fluorescens against Fusarium oxysporum f.sp.nievum isolated from soil samples in Palestine. Journal of Plant stud. 2017;6(2).. doi:10.5539/ jps. v6n2p1 URL: https://doi.org/10.5539/jps.v6n2p1.
CrossRef - Saravanan T., Muthusamy M., Marimuthu T. Development of integrated approach to manage the Fusarial wilt of banana. Crop Protect. 2003;22:1117–1123.
CrossRef - Singh A., Singh H. B. Control of collar rot in mint (Mentha spp.) caused by Sclerotium rolfsii using biological means. Current Sci. 2004;87:362-366.
- Sivan A., Chet I. Biological control of Fusarium spp. in cotton, wheat and muskmelon by Trichoderma harzianum. J. Phytopathol. 1986;116:39–47.
CrossRef - Sohail M., Ahmad A., Khan S. A. Production of cellulases from Alternaria sp. MS28 and their partial characterization. Pak. J. Bot. 2011;43(6):3001-3006.
- Suga H., Karugia G.W., Ward T., Gale L.R., Tomimura K., Nakajima T., Miyasaka A., Koizumi S., Kageyama K., Hyakumachi, M. Molecular characterization of the Fusarium graminearum species complex in Japan. Phytopathol. 2008;98:159-166.
CrossRef - Talaviyan J. R., Jadeja K. B. Efficacy of bioagents alone and in combination microbial population against the wilt incidence of cumin Journal of Biological Con. 2015;29(3):162-166.
- Taylor N. G. Cellulose biosynthesis and deposition in higher plants.New Phytol. 2008;178:239–252.
CrossRef - Thangavelu R., Gopi M. Field suppression of Fusarium wilt disease banana by the combined application of native endophytic and rhizospheric bacterial isolates possessing multiple functions. Phytopathologia Mediterr. 2015;54(2):241−252.
- Thilagavathi R., Saravanakumar D., Ragupathi N., Samiyappan R. A combination of biocontrol agents improves the management of dry root rot (Macrophomina phaseolina) in greengram. Phytopathol Mediter. 2007;46(2):157167.
- Tu C. C., Chang Y. H. Soil microbial activity in relation to Fusarium wilt suppression soils and conducive soil. In: Proc Republic of China–Federal Republic of Germany Seminar Plant Nutrition Soil Sciences. National Science Council, Taipei 1983;189-196.
- Wahid O. A. A. Improving control of Fusarium wilt of leguminous plants by combined application of biocontrol agents. Phytopathol. Mediterr. 2006;45:231-237.
- Wang G., Michailides T. J., Bostock R. M. Improved detection of polygalacturonase activity due to Mucor piriformis with a modified Dinitrosalicylic Acid Reagent. Phytopathol. 1997;87:161-163.
- Wheeler H. Plant pathogen. 1975;2-3. Acad. Press, New York & London.
CrossRef - Zhang J. B., Li H. P., Dang F. J., Qu B., Xu Y. B., Zhao C. S., Lia Y. C. Determination of the trichothecene mycotoxins chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade. Mycol Res. 2007;111(8):967-975.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





