How to Cite | Publication History | PlumX Article Matrix
Effect of Enhanced UV-B Radiation on Germination and Biochemical Components of Maize (Zea Mays L.)
Nagendram Erram1, Anil Gaddameedi2, Swapna Siddamalla1, Tumu Venkat Reddy1 and Manjula Bhanoori1
1Department of Biochemistry, Osmania University, Hyderabad, India.
2Department of Genetics, Osmania University, Hyderabad, India.
Corresponding Author E-mail: bhanoorim@yahoo.co.in
DOI : http://dx.doi.org/10.13005/bbra/2522
ABSTRACT: In the present investigation, Maize seeds of hybrid NK 6240 and 900M Gold were exposed to UV-B (280-320 nm) for periods of 40 and 60 minutes and compared with the control without exposer to UV-B. The biochemical changes associated with UV-B induced resistance were investigated by determination of proline concentration, total soluble sugars, total soluble protein, malondialdehyde content and free amino acids from leaves and roots. Also comparison for germination percentage between control and treated seeds was carried along with biochemical traits. Analysis of variance explains both the hybrids were significantly different in germination percentage, total soluble sugars, proline and free amino acids. Whereas both treatments showed high significant variation for all the studied traits, but genotype × treatment interaction was non-significant for all the traits. 40 minutes UV-B treated seeds showed reduced total soluble sugars and increased malondialdehyde, proline and total soluble protein content. In 60 minutes UV-B treatment, decrease in free amino acids, proline, percentage of germination, and total soluble protein and increase in total soluble sugars was observed.
KEYWORDS: Maize; Proline Total Soluble Sugars; Total Soluble Protein: UV Radiation;
Download this article as:| Copy the following to cite this article: Erram N, Gaddameedi A, Siddamalla S, Reddy T. V, Bhanoori M. Effect of Enhanced UV-B Radiation on Germination and Biochemical Components of Maize (Zea Mays L.). Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Erram N, Gaddameedi A, Siddamalla S, Reddy T. V, Bhanoori M. Effect of Enhanced UV-B Radiation on Germination and Biochemical Components of Maize (Zea Mays L.). Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27510 |
Introduction
Maize (Zea mays L.) is the third most important cereal crop in the world after wheat and rice. The superior position of maize is due to its wide and variety utilization. During the centuries maize plant was known for multifarious uses. Maize is used like a food, fodder for livestock, for producing alcohol and non alcohol drinks, built materials like a fuel and like medicinal and ornamental plant.1-3
UV-B effects plant development, growth and productivity4. Still, a low number of investigations considered the possible subject of UV on induced resistance in plant against biotic and abiotic stress.5-6
The responses of plants to UV irradiation include physiological, biochemical, and morphological changes. In general, UV radiation deleteriously affects plant growth, reducing leaf size, determining the field available for solar energy capture. These determinations have been accomplished primarily through studies in the greenhouse and exposure to artificial sources of UV radiation, extrapolation to changes on crop production as a result of increase in terrestrial solar UV radiation is difficult. Tolerant plants have evolved mechanisms to overcome these harmful changes. The outcomes of most subject fields have shown that resistance to abiotic stresses is usually correlated with a more efficient antioxidant system.7 Also, proline accumulation is seen as a biomarker for plant tolerance to drought and salt stresses due to its osmoprotective function.8 Thus, the present investigation is aimed to examine the force of different doses of UV-B on the antioxidant systems in Maize seeds and biochemical changes and the level of UV stress tolerance.
Materials and Methods
The experiment was conducted at the Department of Biochemistry of the Osmania University. Maize seeds were irradiated using a UV hood source of 125W (15PSIG) for 40 and 60 minutes.
Seed Material
The popular maize hybrids NK 6240 and 900M Gold were used for study as these hybrids are widely accepted by farmers due to high yield and stability.
UV-B Treatment
UV irradiation is an important stress factor for plants resulting in damage to genetic system and cell membranes and various metabolic processes. UV-B has greater damaging effects on plants because the cell macromolecules like DNA and protein have a strong absorption at 280-320 nm. The seeds were exposed to UV-B radiation (280-320 nm) from an artificial source situated at 20 cm distance over the seeds. Durations of exposure time were 40 and 60 min. After treatment, petridishes containing the germinated seeds were enwind with aluminum foil until sowing to minimize any possible photo reactivation processes.9-10
Biochemical Analysis
The leaves of 10th day seedlings were collected from each treatment for the analysis of protein11, Malondialdehyde (MDA), Proline, Free amino acids (FAA), and Total soluble sugars (TSS). Protein estimation was carried out by Lowry method11 and MDA was analyzed by Lipid Peroxidation method,12 Proline and FAA were analyzed by Ninhydrin method.13 Total Soluble Sugars was analyzed by the Phenol Sulphuric Acid method.14
Statistical Analysis
The data are shown as mean ± SE from five replicates. Data was subjected to two-way ANOVA to examine the issue of seed treatment with different dosages of UV-B irradiation on the grown plants and the levels of significance are represented by *P ≤ 0.05, **P ≤ 0.01, and non significant (NS) and combined means of both the hybrids were compared using Duncan’s multiple range test.
Results and Discussion
The amount of UV-B radiation reaching to earth surface is increasing. Plants which use sunlight for photosynthesis cannot avoid the exposer to enhanced level of UV-B radiation and they are at risk. Hence understand the effect of UV-B radiation on various biochemical parameters in Maize would be useful to researchers to breed maize crop according to current climate change conditions. Two popular Maize hybrids were studied to understand the impact of UV-B radiation on Maize seed germination and biochemical parameters. Two factor analysis of variance showed significant difference for UV-B treatment in all the studied traits, whereas two hybrids were significantly different in Germination, TSS, Proline content and FAA. The Genotype × Treatment interactions were non-significant for all traits (Table 1).
Table 1: Analysis of variance for genotypes, treatment and Genotype × Treatment interactions.
| Germination | MDA | TSS | Proline | FAA | Protein | |
| Genotype | 34.72* | 0.25NS | 6384.5** | 1.3* | 0.00092** | 0.057NS |
| Treatment | 751.38** | 24.85** | 115211.05** | 26.92** | 0.025** | 92.65** |
| G x T | 10.05NS | 1.32NS | 393.16NS | 0.573NS | 0.000078NS | 0.53NS |
| C.V | 2.74 | 15.42 | 4.32 | 5.73 | 11.77 | 5.54 |
| L.S.D | 2.65 | 1.41 | 13.63 | 0.49 | 0.0098 | 0.8316 |
MDA= Malondialdehyde, TSS= Total Soluble Sugars, FAA= Free Amino Acids
*Significant at 5% level, ** Significant at 1% level, NS non-significant.
Germination
Both treatments showed reduce germination, although both treatments were significantly different compared to control 40 minute UV-B treatment showed less reduction in germination compared to 60 min UV-B treatment (Table 3 and Figure 1A).
Table 2: Mean values for two hybrids with different treatments.
| NK 6240 | 900M Gold | |||||
| control | 40Min UV-B | 60 Min UV-B | control | 40 Min UV-B | 60 Min UV-B | |
| Germination | 100 | 100 | 80 | 100 | 93.33 | 78.33 |
| MDA | 7.65 | 11.72 | 7.17 | 7.68 | 10.43 | 7.71 |
| TSS | 154.6 | 273.3 | 416.3 | 174 | 317 | 466.3 |
| Proline | 7.54 | 11.33 | 6.81 | 7.16 | 10.11 | 6.3 |
| FAA | 0.143 | 0.028 | 0.045 | 0.163 | 0.035 | 0.061 |
| Protein | 12.1 | 18.6 | 12.4 | 12.1 | 19 | 11.6 |
MDA= Malondialdehyde, TSS= Total Soluble Sugars, FAA= Free Amino Acids
Malondialdehyde (MDA)
For 40 min UV-B treatment both the hybrids showed enhanced level of MDA compared to control (Figure 1B).There was no significant difference observed between control and 60 minute UV-B treated seeds (Table 3).This notice was previously found in leaves of Arabidopsis challenged with the fungus Botrytis where MDA levels were correlated with the survival of tissues. The results suggest that trienoic fatty acids (the major precursors of MDA) contribute to ROS control via non enzymatic oxidation.15
Table 3: Effect of UV-B treatment on biochemical traits compared using Duncan’s multiple range test.
| Treatment | Germination | MDA | TSS | Proline | FAA | Protein |
| Control | 100a | 7.66 b | 164.33 c | 7.34 b | 0.152 a | 12.06 b |
| UV-B (40 min) | 96.66b | 11.07 a | 295.16 b | 10.71a | 0.031 c | 18.82 a |
| UV-B (60 Min) | 79.167 c | 7.44 b | 441.33a | 6.8 b | 0.053 b | 11.97 b |
Values a, b, c represent significant differences compared to controls at P < 0.05 according to Duncan’s multiple range test
Total Soluble Sugars (TSS)
The TSS results were observed in UV-B seed treatment grown plants at the doses of 40 and 60 min. The highest increment (~ 3.5-fold increase) was observed at the dosage of 60 min followed by a dose of 40 min which led to a ~ 1.5- fold increase over the control (Figure1C), Duncans multiple range test showed both the treatments significantly differ from control. In both hybrids UV-B treated plants had a universal propensity to lower sink capacity.16
Proline Concentration
Proline concentration increased in both hybrids in 40 min treatment, whereas in 60 min treatment there was no significant change observed compared to control (Figure 1D). Proline has many roles in water stress resistance, being an osmoregulator proline also protects protein from damage and enhances enzyme activities.17-19 High levels of proline synthesis maintain the NADP+/NADPH ratio in the cell ascities under normal conditions.20 In response to UV-B stress, FAA content was declined in 40 and 60 min compared to the control (Figure1E).In previous studies a dose dependent decrease in the triacylglycerol content and a concomitant increase in FAA content were observed after UV-irradiation in nutmeg.21
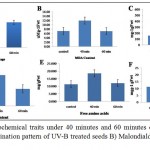 |
Figure 1: Studied biochemical traits under 40 minutes and 60 minutes of UV-B treatment in maize seeds A) Germination pattern of UV-B treated seeds B) Malondialdehyde content in UV-B treated seeds C)
|
Total soluble sugars levels in UV-B treated seeds D) Proline concentration in UV-B treated seeds E) FAA content in UV-B treated seeds and F) Total soluble proteins in UV-B treated seeds.
Total Soluble Proteins
Plants produced from UV-B treated seeds for 40 min showed a considerable elevation in total soluble protein levels as compared to control plants, whereas 60 min UV-B treated seeds were on par with control (Figure 1F). Physiological and biochemical processes in plants are significantly affected by UV irradiation. The irradiation of seeds with high dosages of UV rays disturbs the synthesis of protein,22 UV-B radiation promoted the aggregation of a novel 66-KD protein in pea, love apple, tobacco and brassica napus.23
Conclusions
In most of the studied biochemical traits 40 minutes of UV-B treatment showed more variation than 60 minutes of UV-B treatment granting to the obtained results, it could be reasoned that the exposure of Maize seeds to UV-B enhanced accumulation of proline in the leaves and root which lessened the effect of osmotic stress. In the present study, we demonstrated that the optimal dose for maize seeds was at 40 min at which achieved the enhanced level of stress tolerance.
Acknowledgement
Nagendram Erram would like to thank University Grants Commission (UGC-BSR-RFMS), India for providing senior research fellowship (SRF).
Conflict of Interest
All authors declare no conflict of interest.
Funding
This work was supported in part from the grant UGC-CPEPA-OU-4 to Dr. Manjula Bhanoori.
References
- Alahdadi I., Oraki H., khajani P. F. Effect of water stress on yield and yield components of sunflower hybrids. Afr J Biotechnol. 2011;10(34):6504-6509.
- Khodarahmpour Z. Effect of drought stress induced by polyethylene glycol (PEG) on germination indices in corn (Zea mays L.) hybrids. Afr J Biotechnol. 2011;10(79):18222-18227.
CrossRef - Bekric V., Radosavljevic M. Contemporary approaches to maize utilization. PTEP (Serbia). 2008.
- Mahdavian K., Ghorbanliand M. K and Kalantari M. The Effects of ultraviolet radiation on the contents of chlorophyll, flavonoid, anthocyanin and proline in Capsicumannuum L. Turk J Bot. 2008;32:25-33.
- Teklemariam T. A., Sparks J. P. Gaseous fluxes of peroxyacetyl NO3. (PAN) into plant leaves. Plant.Cell and Environment. 2004;27:1149-e 1158.
- Stevens C., Khan V. A., Lu J. Y., Wilson C. L., Pusey P. L., Kabwe M. K., Igwegbe E. C. K., Chalutz E and Droby S. The effects of low-dose ultraviolet light-C treatment on poly galacturonase activity, delay ripening and Rhizopus soft rot development of tomatoes. Crop Protection. 2004;23:551–554.
CrossRef - Meratan A. A., Ghaffari S. M and Niknam V. Effects of salinity on growth, proteins and antioxidant enzymes in three Acanthophyllumspecies of different ploidy levels. JUST. 2008;33(4):1-8.
- Huang Y. Z., Bie Z., Liu A., Zhen A and Wang W. The Protective role of proline against salt stress is partially related to the improvement of water status and peroxidase enzyme activity in cucumber. Soil Science and Plant Nutrition. 2009;55:698-704.
CrossRef - Stevens C., Khan V. A., Lu J. Y.,Wilson C. L., Pusey P. L., Kabwe M. K., Igwegbe E. C. K, Chalutz E and Droby S. The germicidal and hermetic effects of UV-C light on reducing brown rot disease and yeast micro flora of peaches. Crop Prot. 1998;17:75-84.
CrossRef - Liu J.C., Stevens V. A., Khan J. Y., Lu C. L., Wilson O. A., Kabwe M. K., Pusey P. L., Chalutz E., Sultana T and Droby S. Application of ultraviolet-C light on storage rots and ripening of tomatoes. J. Food .Prot. 1993;56:868-872.
CrossRef - Lowry O. H., Rosenbrough N. J., Farr A. L and Randall R. J. Protein measurement with the Folin Phenol Reagent .J. Biol.Chem. 1951;193;256-275.
- Heath R. L and Packer L. photoperoxidation in isolated chloroplasts I., Kinetics and stoichiometry of fatty peroxidation. Arch Biochem.Biophys. 1968;125:189-198.
CrossRef - Bates L. S., Waldern R. P., Teare I. D. Rapid determination of free proline for water stress studies. Plant and Soil. 1973;39:205-207.
CrossRef - Hadadchi G. H. Plant chemistry and physiology. Jahad Daneshgahi Mazandaran, Natural Resources University, first edition (in farsi). 1987.
- Mène-Saffranè L.,Dubugnon L.,Chételat A., Stolz S., Gouhier-Darimont C and Farmer E. E. Non enzymatic oxidation of trienoic fatty acids contributes to reactive oxygen species management in Arabidopsis. J. BiolChem. 2009;284(3):1702–1708.
CrossRef - Correia C. M., Areal E. L.V., Torres-pereira M. S and Torres-pereira J. M. G. Intra specific variation in sensitivity to ultraviolet-B radiation in maize grown under field conditions. Growth and morphological aspects. Field Crops Res. 1998;59:81-89.
- Ambikapathy J., Marshall J. S., Hocartand C. H and Hardahm A. R. The role of proline in Osmo regulation in Phytophthoranicotianae. Fungal genetics and biology. 2002;35(3):287-299.
CrossRef - Sharma P and Dubey R. S. Modulation of nitrate reductase activity in rice seedlings under aluminium toxicity and water stress: role of Osmolytes as enzyme protectant. J. PlantPhysiol. 2005;162(8):854-864.
CrossRef - Mishra S and Dubey R. S. Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: role of proline as enzyme protectant. J. Plant Physiol. 2006;163(9):927-936.
CrossRef - Hare P. D and Cress W. A. Metabolic implications of stress- induced proline accumulation in plants. Plant Growth Regul. 1997;21:79–102.
CrossRef - Niyas Z., Prasad S., Achyut V., Gholap S and Sharma A. Effect of γ-Irradiation on the Lipid Profile of Nutmeg (MyristicafragransHoutt.).Food Technology Division, Bhabha Atomic Research Centre, Trombay, Mumbai 400 085, India. J. Agric Food Chem. 2003;51(22):6502–6504.
CrossRef - Xiuzher L. Effect of irradiation on protein content of wheat crop. J. Nucl. Agricul. Sci.China. 1994;15:53-5.
- Wilson R., Franch R., Wilson C., Amarasinghe S., Anderson J., Tjiang S., Liao S. W., Tseng C. W.,Hall M., Lam M and Hennessy. J. An Overview of the SUIF Compiler System. 1995.

This work is licensed under a Creative Commons Attribution 4.0 International License.





