How to Cite | Publication History | PlumX Article Matrix
Formulation and Characterization of Kaffir Lime Oil Nanoemulsion
Ghea Putri Christy1 , Dewa Ayu Arimurni2
, Dewa Ayu Arimurni2 , Made Dwi Pradipta Wahyudi2
, Made Dwi Pradipta Wahyudi2 , Ronny Martien3
, Ronny Martien3 and Woro Anindito Sri Tunjung1
and Woro Anindito Sri Tunjung1
1 Laboratory of Biochemistry, Faculty of Biology, Universitas Gadjah Mada, Jalan Teknika Selatan, Sekip Utara, Yogyakarta 55281, Indonesia.
2School of Pharmacy Mahaganesha, Jalan Tukad Barito Timur No. 57, Renon, Denpasar Selatan, Denpasar, Bali 80226, Indonesia.
3Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia.
Corresponding Author E-mail: wanindito@ugm.ac.id
DOI : http://dx.doi.org/10.13005/bbra/2525
ABSTRACT: Kaffir lime oil has many health benefits. However, an obstacle to its commercial use is oxidation during storage. Nanoemulsions (particulate colloidal systems) have been shown to be suitable carriers for lipophilic essential oil constituents due to amphipathic compounds that facilitate solubility. The objectives of this study were to formulate thermodynamically stable kaffir lime oil nanoemulsions and to investigate their physicochemical properties. Air-dried leaves of kaffir lime were subjected to steam distillation to obtain essential oil. Preparation of nanoemulsions was done using the spontaneous emulsification method. Tween 80 and propylene glycol were selected as surfactant mix components. The oil phase consisted of Miglyol 812 as a carrier oil for kaffir lime oil while double-distilled water was used in the aqueous phase. The best formula with transmittance above 95% and highest essential oil content was selected. It contained 20% of Tween 80, 10% of propylene glycol, 1.25% Miglyol 812, and 3.75% kaffir lime essential oil. This formula was then characterized and its thermodynamic stability determined. . The results showed that kaffir lime oil nanoemulsions were thermodynamically stable and robustly withstood variations in temperature, centrifugation, and long-term storage. Additionally, the nanoemulsions had low viscosity, which may facilitate its development as a pharmaceutical compound.
KEYWORDS: essential oil; kaffir lime;kaffir lime; Nanoemulsions; spontaneous emulsification
Download this article as:| Copy the following to cite this article: Christy G. P, Arimurni D. A, Wahyudi M. D. P, Martien R, Tunjung W. A. S. Formulation and Characterization of Kaffir Lime Oil Nanoemulsion. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Christy G. P, Arimurni D. A, Wahyudi M. D. P, Martien R, Tunjung W. A. S. Formulation and Characterization of Kaffir Lime Oil Nanoemulsion. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=26797 |
Introduction
Kaffir lime (Citrus hystrix D.C.) is well known for its distinctive lemon fragrance and astringent flavor. Kaffir lime has been used for many purposes, including traditional medicines, a spice in asian cookery, and an ingredient in perfumery 1
The efficacy of kaffir lime as a medicinal plant results from its secondary metabolite content. A number of volatile compounds found in kaffir lime are reported to have bioactive properties that may be useful in promoting health and fighting disease. Citronellal, which is the most abundant volatile compound in kaffir lime leaf, shows antibacterial activities towards Moraxella catarrhalis, Haemophilus influenza, Staphylococcus pneumonia, S. aureus and Acinetobacter baumannii.2 Another major volatile compound in the leaf, β-citronellol, has been shown to be a potential inhibitor of HIV-1 RT and is also popular for aromatherapy.3,4 Limonene in kaffir lime leaf contributes to the anti-inflammatory activity of kaffir lime oil against Propionibacterium acnes, a skin commensal bacteria which causes the chronic skin disease acne vulgaris.5 In addition, limonene can promote wound-healing and reduce post-acne scarring and help to remove acne blemishes.6 All of these volatile compounds are generally obtained from essential oil extraction. Other terpenoids such as citronellyl acetate, sabinene, nerolidol, and linalool are also found in kaffir lime leaf essential oil at lower concentrations.7,8 Furthermore, an in vitro study has suggested the use of kaffir lime leaf essential oil as an anti-cancer compound. The essential oil possesses anti-proliferative activity against human mouth epidermal carcinoma and murine leukemia cell lines.9
However, kaffir lime essential oil needs to be improved because it tends to be easily oxidized in the presence of light, oxygen, increasing acidity, heavy metals, water, and increasing heat.10 As water makes up most of human body and oxygen is present in bloodstream, essential oil is prone to hydrolysis and oxidation when administrated to the body. Moreover, the highly lipophilic nature of essential oil constituents decreases its bioavailability in the human body. The lipophilic essential oil is essentially insoluble in blood and thus must be reduced in concentration prior to administration, thus greatly limiting the clinical utility of kaffir lime-derived compounds.11
Nanoemulsion is a type of nanometric carrier system in which particles are less than 100 nm in droplet size, consisting of oil, water, and surfactant.12,13 This particulate colloidal system is described as suitable carrier for lipophilic bioactive compound delivery, including essential oil constituents, due to its amphiphilic behavior. Moreover, it has several unique properties due to its small droplet size such as optical transparence, long-term stability, and enhanced bioavailability.14 Furthermore, a nanoemulsion system is easy to prepare using the spontaneous emulsification method [12]. Various kind of essential oils have been successfully formulated into nanoemulsions such as orange oil, zedoary turmeric oil, and oregano essential oil.15, 16,17 As other essential oils mentioned, kaffir lime essential oil could be also formulated into nanoemulsion and this method might be a promising strategy to improve the effectiveness of the kaffir lime essential oil bioactivity for medicine and pharmaceutical use.
This is the first reported study in formulation and characterization of kaffir lime oil nanoemulsion. The objectives of this study were to formulate a thermodynamically stable kaffir lime oil nanoemulsion and to investigate its physicochemical properties.
Materials and Methods
Essential oil Preparation
Fresh kaffir lime leaves were collected from Candirejo Village, Borobudur District, Magelang Regency, Central Java. The leaves were washed with tap water and air-dried using fan for two weeks (until dry weight of the leaves are in constant level). Dried leaves then weighed and chopped prior to steam distillation. Steam distillation was done for four hours. Sodium sulfate anhydrous (e-Merck) was used to separate the obtained distillate from water.
Nanoemulsion Preparation
Nanoemulsion was prepared using spontaneous emulsification method.16 First, surfactant Tween 80 (e-Merck) and co-surfactant propylene glycol (e-Merck) were homogenized with vortex. This mixture is called surfactant mix. Oil phase, consists of Miglyol 812 (Sigma) as carrier oil and kaffir lime leaf essential oil, was added into surfactant mix afterwards and homogenized with vortex. The mixture of surfactant mix and oil phase, namely organic phase, was then added drop by drop into aqueous phase (citrate buffer or double distilled water) using pipette with rate at about 2 mL/minute while stirred with magnetic stirrer at 500 rpm. All steps were done at room temperature.
Screening of Best Nanoemulsion Formula
Screening of the best formula was done based on the acquired data of percent transmittance. Each designed formula was subjected to transmittance measurement against distilled water using UV-Vis spectrophotometer at 650 nm. Formula with percent transmittance number above 95 % was selected as the best formula.18
Physicochemical Characterization
In addition to transmittance measurement, physicochemical characteristics of the best formula were observed by viscosity measurement and particle size analysis. The viscosity of nanoemulsion was measured using cone and plate model Rheosys viscometer at 50, 62.5, 75, 87.5, and 100 rpm. Particle size analysis of nanoemulsion was done with Horiba SZ-100 particle size analyzer.
Thermodynamic Stability Test
Thermodynamic stability of the nanoemulsion was examined with freeze-thaw test, centrifugation test, and storage duration test. Nanoemulsion was subjected to freeze-thaw test for six cycles; each cycle consists of 24 hours storage at -20°C and 24 hours storage at 25°C. Physical destabilization such as creaming, cracking, aggregation, and sedimentation of nanoemulsion were observed after six cycles. Centrifugation test was done after freeze-thaw test. Nanoemulsion was centrifuged for 30 minutes at 3000 rpm. For storage duration test, nanoemulsion was stored for 28 days at room temperature. After this test, physical destabilization was observed.
Results and Discussion
Previous studies suggest that the best surfactant, co-surfactant, and oil phase for oil in water nanoemulsions are Tween 80, propylene glycol, Miglyol 812, respectively [16, 19, 20]. All of these components are food grade and thus safe to be added as excipients in pharmaceutical products. Therefore we designed a carrier formula using these components as shown in Table 1.
Table 1: Carrier nanoemulsions formulae
| Number | Formula | Transmittance (%) | Physical appearance | Category | ||||
| Surfactant | Co-surfactant | Oil Phase | Aqueous Phase | |||||
| Tween
80 |
Propylene Glycol | Miglyol
812 |
Citric acid buffer | Double-distilled water | ||||
| 1 | 16.67% | 8.33% | 10% | 65% | 0.1 | Cloudy | – | |
| 2 | 18% | 9% | 5% | 68% | 0.5 | Cloudy | – | |
| 3 | 18.75% | 6.25% | 10% | 65% | 0.6 | Cloudy | – | |
| 4 | 20.25% | 6.75% | 5% | 68% | 0.7 | Cloudy | – | |
| 5 | 20% | 10% | 5% | 65% | 71.9 | Transparent | – | |
| 6 | 20% | 10% | 5% | 65% | 87.6 | Transparent | – | |
| 7 | 20% | 10% | 4% | 66% | 97.6 | Transparent | + | |
Note: + Classified as nanoemulsion (percentage of transmittance > 95 %)
Emulsion
Bold typed formula identified as most effective preparation
Since the carrier formula will be used to encapsulate kaffir lime oil, Tween 80 and propylene glycol as carrier components have more advantages than other surfactants and co-surfactants
considering their chemical structure and physicochemical nature. Tween 80 was selected as a surfactant in the organic phase because it is less toxic, stable under in vivo conditions, has biological compatibility, is not significantly affected by the potential of hydrogen, and readily available. Long alkyl chain length of Tween 80 (C18) provides greater solubilisation capacity for hydrophobic solutes than other shorter alkyl chain-length Tween such as Tween 20 (C12). Meanwhile, propylene glycol is an amphiphilic molecule that has ability to increase solubility between oil phase and aqueous phase. It also takes a role as co-surfactant when the surfactant has a low concentration in the nanoemulsions system.12,21,22 Moreover, a previous study showed that disruption in the interfacial region can be minimized when the hydrocarbon chain length of surfactant is equal to the sum of co-surfactant chain length and oil chain length.23 Propylene glycol (C3H8O2) has a short chain length while Miglyol 812 (carrier oil) has medium-chain length.24,25,26 Therefore Tween 80 (C64H124O26) with long-chain length was able to entrap propylene glycol and Miglyol 812, prior to nanocarrier droplet formation.27 In Table 1, double-distilled water and citric acid buffer were used as the aqueous phase, for the purpose of comparing the carrier nanoemulsions’ behaviors in acidic solutions and neutral solutions. The results showed that double-distilled water tends to produce more transparent nanoemulsions than citric acid buffer. Consequently, double-distilled water was chosen as the best aqueous phase as it is also more closely matches a physiological aqueous environment. Based on Table 1, we found that increasing the concentration of surfactant mix (Tween 80 and propylene glycol) has a tendency to increase the transmittance value in nanoemulsions. However, the concentration of surfactant has to be low enough to avoid any possible side effect such as hypersensitivity reactions.28 Hence formula number 6 and 7 were chosen as the best carrier formula as its surfactant mix concentration was in an acceptable range yet maintained the highest percentage of transmittance. Formula 6 has lower transmittance than 95%, but it is still considered as a potential carrier for kaffir lime nanoemulsions because the addition of essential oils could increase the transmittance. Those carrier formulas were then incorporated with kaffir lime essential oil, showed in Table 2.
Table 2: Kaffir lime oil nanoemulsions formula
| Number | Formula | Transmittance (%) | Physical appearance | Category | ||||
| Surfactant | Co-surfactant | Oil Phase | Aqueous Phase | |||||
| Tween
80 |
Propylene Glycol | Miglyol
812 |
Essential oil | Double-distilled water | ||||
| 1 | 20% | 10% | 1% | 3% | 66% | 99.8 | Transparent | + |
| 2 | 20% | 10% | 1.25% | 3.75% | 65% | 97.53 | Transparent | + |
Note: + Classified as nanoemulsion (percentage of transmittance > 95 %)
Emulsion
Bold typed formula is the best formula because it contents higher kaffir lime oil concentration
Table 3: Freeze-thawing test results of kaffir lime oil nanoemulsions.
| Day 0 | Day 12 (after six cycles) | Category | ||
| Transmittance (%) | Physical appearance | Transmittance (%) | Physical appearance | |
| 95.4 ± 0.0 | Yellowish, transparent | 95.8 ± 0.9 | Yellowish, transparent | Stable |
Note: Classified as nanoemulsion if the percentage of transmittance > 95 %.
The formula is stable if its transmittance remained at > 95 % after six cycles.
The optically transparent formula indicated that the nanoemulsions have smaller droplet size than visible light wavelength [29]. Based on transmittance measurements and essential oil content, the best formula chosen was with 20 % of Tween 80, 10% of propylene glycol, 1.25 % Miglyol 812, and 3.75% of kaffir lime essential oil. This formula has the highest essential oil content with transmittance more than 95%. Physical stability of the chosen formula then observed and the result is showed in Table 3.
Table 3 shows that the transmittance of the chosen formula has a quite constant value from day 0 to day 12, after the sixth cycle of freeze-thawing was done. Moreover, physical appearance of the nanoemulsions did not change after six cycles of freeze-thawing. The kaffir lime oil nanoemulsions still had a yellowish transparent appearance. This indicates that the chosen formula is stable from temperature changes. Yellowish color of nanoemulsions was coming from tween 80 color and the natural color of kaffir lime oil. Table 3 also showed a slight increase in transmittance after freeze-thawing. The increasing percentage might be caused by decreasing interfacial tension during freeze and thawing. As the interfacial tension decreases, the droplet size become smaller and nanoemulsions become more transparent.
Table 4: Stability of kaffir lime oil nanoemulsions after 28 days of storage
| Transmittance (%) | Physical appearance after 28 days storage | Category | ||
| Day 0 | Day 14 | Day 28 | ||
| 95.4 ± 0.0 | 95.9 ± 0.3 | 96.4 ± 0.4 | Yellowish, transparent | Stable |
Note: Classified as nanoemulsion if the percentage of transmittance > 95 %.
Storage stability of kaffir lime nanoemulsions was observed in room temperature at day 0, day 14 and day 28. The transmittance measurement and physical appearance of the nanoemulsions is shown in Table 4.
Table 5: Physical destabilization of kaffir lime oil nanoemulsion after stability tests
| Type of
stability test |
Observed destabilization | Category | |||
| Creaming | Cracking | Sedimentation | Aggregation | ||
| Freeze-thawing | – | – | – | – | Stable |
| Storage duration | – | – | – | – | Stable |
| Centrifugation | – | – | – | – | Stable |
Note: + detected after the stability test
not detected after the stability test
The formula is stable if creaming, cracking, sedimentation and aggregation are not detected after the stability tests.
Based on Table 4, the transmittance was slightly increased from day 0 to day 28. This phenomenon also might be caused by decreasing interfacial tension between oil phase and aqueous phase. Furthermore, the nanoemulsions still have yellowish, transparent appearance and have transmittance > 95 % even after 28 days. This indicated that the formula is stable for a quite long duration of storage. Next, physical destabilization of the nanoemulsion from three stability tests is show in Table 5.
Creaming is movement of dispersed droplets to upper layer of aqueous phase. Conversely, dispersed droplets movement to lower layer of aqueous phase is called sedimentation. Aggregation occurs when dispersed droplets become close one to another without losing their individual intergrity. Cracking is the coalescence of nanoemulsions droplets.30 Table 5 showed that the chosen formula has no physical destabilization after each stability test done thus the formula is physically stable.
The characteristics of each formula component contribute to the physical stability. One criteria to determine the ability of surfactant to act as an emulsifier in the oil phase is hydrophilic-lipophilic balance (HLB) value. Surfactant with a HLB value more than 11 means the surfactant is hydrophilic and fit to be used in oil-in-water system.31 The HLB value of Tween 80 is 15.32 This HLB value shows that Tween 80 is suitable to be used in kaffir lime oil nanoemulsion which has 65 % of water component. Moreover, high concentration of surfactant is needed to obtain an optimum formula with spontaneous emulsification. In order to stabilize the formula, required surfactant concentration is more than 30% in general.33 Nevertheless, in this study 20% of Tween 80 is enough to make a physically stable oil-in-water nanoemulsion. This is caused by propylene glycol as co-surfactant. Co-surfactant has a role in decreasing interfacial tension and increasing interfacial fluidity to keep the nanoemulsions stable.12 The density of the oil phase also has an impact in physicochemical nature of nanoemulsions. Oil-in-water nanoemulsions with oil that has density value under 1 g/mL tend to resist creaming.34 The components of the oil phase in this study, Miglyol 812 and kaffir lime essential oil, have density values of 0.95 g/mL and 0.86 g/mL respectively.2,35 This density value likely contributed to the nanoemulsions stability.
Table 6: Physical destabilization of kaffir lime oil nanoemulsion after stability testsViscosity of kaffir lime oil nanoemulsions at various centrifugation speeds.
| Viscometer speed (rpm) | Viscosity (mPa.s) | |
| 50.0 | 146.6 | |
| 62.5 | 142.7 | |
| 75.0 | 135.9 | |
| 82.5 | 130.8 | |
| 100.0 | 124.2 | |
Next, physicochemical nature of the chosen formula was further observed. Viscosity measurements were conducted and the results are shown in Table 6.
In order to enable the production of nanoemulsions in capsule form, the nanoemulsions need to have viscosity value of 100 to 25,000 mPa.s.36 Based on Table 5, the viscosity of the best formulation is consistent with usage in capsule applications. This low viscosity might be caused by Miglyol 812 and kaffir lime oil which have low viscosities.22 The viscosity of the nanoemulsions could be increased by addition of oil with long-chain fatty acid into the oil phase. Nonetheless, together with the nanoemulsion’s translucent appearance, this low viscosity may be more appealing asthetically. The low viscosity also can be improved thus the chosen formula and could be made into roll-on type formulations, sprays and gels.25
Another criteria is the particle size distribution of the nanoemulsions. The distribution graphic of nanoemulsions particle size is shown on Figure 1.
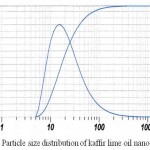 |
Figure 1: Particle size distribution of kaffir lime oil nanoemulsions
|
The average size of droplets in the chosen formula is 18.23 ± 0.12 nm with a polydispersity index of 0.36 ± 0.01. These results confirmed that the size of obtained nanoemulsions is less than 100 nm.13 Tween 80 contributed to this small droplet size. With high HLB value, tween 80 was able to stabilize the particle in the oil-in-water system, thus decreasing droplet size. Small droplet size also decreased physical destabilization risk during storage. Therefore kaffir lime oil nanoemulsions still have transparent appearance after 28 days of storage (Table 3). The polidispersity index shows particle size distribution in a scale 0 to 1. The higher the podispersity index value of nanoemulsions, the less homogenous are its particle size distribution. Most of our nanoemulsions have a polidispersity index value of 0.3. The chosen formula has polidispersity index of 0.36 and this indicated that the formula is polydispersed.22
In conclusion, kaffir lime oil can be formulated into nanoemulsions using Tween 80, propylene glycol, Miglyol 812, and double-distilled water. Our kaffir lime oil nanoemulsions are thermodynamically stable, and robustly resist changes from variations in temperature, centrifugation, and long-term storage. The best candidate formulation was chosen from our results. This formulation had low viscosity, suggesting strong potential as a candidate compound for pharmaceutical production. Optimizing this nanoemulsion formula and increasing its kaffir lime content may permit its use as an antibacterial, anti-inflamatory, and/or aromatherapy agent.
Acknowledgements
We would like to thank The Ministries of Research, Technology, and Higher Education Republic of Indonesia for funding our research through Penelitian Unggulan Perguruan Tinggi grant (to W.A.S.T) and BiNDR research group for providing nanoemulsion materials.
References
- Abirami A., Nagarani G., Siddhuraju P. The medicinal and nutritional role of underutilized citrus fruit Citrus hystrix (Kaffir lime) a review. Drug Invention Today. 2014;6(1):1–5.
- Srisukh V., Tribuddharatb C., Nukoolkarnc V., Bunyapraphatsarac N., Chokephaibulkitd, Phoomniyomb K. S., Chuanphungb S., Srifuengfung S. Antibacterial activity of essential oils from Citrus hystrix (makrut lime) against respiratory tract pathogens. Science Asia. 2012;38:212–17.
CrossRef - Mori K., Obossou E. K., Suwa S., Miura S., Oh S., Jinbo N., Ishibashi Y., Shikamoto Y., Hosono T., Toda T., Tomobe K., Shinozuka T., Nakajo S. Human Immunodeficiency virus type 1 (hiv-1) reverse trans criptase inhibitory effect of Cymbopogon nardus essential oil. International Journal of Advanced Research in Botany. 2016;2(1):7–13.
- Vasconcelos T. B., Ribeiro-Filho H. V., Lucetti L. T., Magalhães P. J. C. B-citronellol an alcoholic monoterpene with inhibitory properties on the contractility of rat trachea. Brazilian Journal of Medical and Biological Research. 2016;49(2):e4800. DOI: 10.1590/1414-431X20154800.
CrossRef - Achermann Y., Goldstein E. J. C., Coenye T., Shirtliff M. E. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clinical Microbiology Reviews. 2014;27(3):419–40.
CrossRef - Lertsatitthanakorn P., Taweechaisupapong S., Aromdee C., Khunkitti. In vitro bio activities of essential oils used for acne control. Int J Aromather. 2006;16:43–49.
CrossRef - Nor O. M. Volatile aroma compounds in Citrus hystrix oil. J. Trop. Agric. Food Sci. 1999;27:225–29.
- Othman S. N. A. M., Hassan M. A., Nahar L., Basar N., Jamil S., Sarker S. D. Essential oils from the malaysian citrus (rutaceae) medicinal plants. Medicines. 2016;3(13). DOI:10.3390/medicines 3020013.
CrossRef - Manosroi J., Dhumtanom P., Manosroi A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines.Cancer Letters. 2006;235(1):114–20.
CrossRef - Turek C., Stintzing F. C. Stability of essential oils: a review. Comprehensive Reviews in Food Science and Food Safety. 2013;12(1):40–53.
CrossRef - Watkins R., Wu L., Zhang C., Davis R. M., Xu B. Natural product-based nanomedicine: recent advances and issues. International Journal of Nanomedicine. 2015;10:6055–74.
- Azeem A., Rizwan M., Ahmad F. J., Iqbal Z., Khar R. K., Aqil M., Talegaonkar S. Nanoemulsion components screening and selection: a technical note.American Association of Pharmaceutical Scientists PharmSciTech. 2009; 10(1):69–76.
CrossRef - Aboofazeli R. Nano metric-scaled emulsions (nano emulsions).Iranian Journal of Pharmaceutical Research. 2010; 9(4):325–26.
- Salvia-Trujillo L., Martín-Belloso O., McClements D. J. Excipient nano emulsions for improving oral bio availability of bio actives. Nano materials. 2016;6(1):1–17.
CrossRef - Zhao Y., Wang C., Chow A. H. L., Ren K., Gong T., Zhang Z., Zheng Y. Self-nano emulsifying drug delivery system (SNEDDS) for oral delivery of zedoary essential oil formulation and bio availability studies. International Journal of Pharmaceutics. 2010;383:170–77.
CrossRef - Chang Y., McClements D. J. Optimization of orange oil nano emulsion formation by is other mal low-energy methods influence of the oil phase, surfactant, and temperature. Journal of Agricultural and Food Chemistry. 2014; 62(10):2306–12.
CrossRef - Moraes-Lovison M., Marostegan L. F. P., Peres M. S., Menezes I. F., Ghiraldi M., Rodrigues R. A. F., Fernandes A.M., Pinho S. C. Nano emulsions encapsulating oregano essential oil Production stability antibacterial activity and incorporation in chicken pâté. LWT – Food Science and Technology. 2017;77:233–40.
CrossRef - Selvam R., Kulkarni P. K., Dixit M. Preparation and evaluation of self-nano emulsifying formulation of efavirenz. Indian Journal of Pharmaceutical Education and Research. 2013;47(1):47–54.
- Widlak N. (ed) Physical Properties of Fats, Oils and Emulsifiers. Illinois American Oil Chemists’ Society Press. 2000.
- Lim T. Y., Poole R. L., Pageler N. M. Propylene glycol toxicity in children. The Journal of Pediatric Pharmacology and Therapeutic. 2014;19(4):277–82.
- Florence A. T., Siepmann J (eds) Modern Pharmaceutics Volume 1 Basic Principles and Systems 5th edn. Boca Raton. CRC Press. 2009;466.
- Grumezescu A. (ed) Emulsions. London Academic Press. 2016;5–9. 92–93, 160–162
- Prieto C., Calvo L. Performance of the bio compatible surfactant Tween 80, for the formation of micro emulsions suitable for new pharmaceutical processing. Journal of Applied Chemistry. 2013;10. Article ID 930356 DOI: 10.1155/2013/930356.
CrossRef - Cognard P. (ed) Handbook of Adhesives and Sealants 1st edn. Vol. 1. Oxford Elsevier Science. 2005;116.
- Date A. A., Desai N., Dixit R., Nagarsenker M. Self-nano emulsifying drug delivery systems formulation insights applications and advances. Nano medicine. 2010;5(10):1595–1616.
CrossRef - Templeton A. C.,Byrn S. R.,Haskell R. J and Prisinzano Th. E. (eds) Discovering and Developing Molecules with Optimal Drug-Like Properties. New York: Springer. 2015;475.
CrossRef - Haynes W. M. (ed) CRC Handbook of Chemistry and Physics 97th edn. Boca Raton CRC Press. 2017;6-198.
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources Added to Food). Scientific opinion on the re-evaluation of polyoxyethylene sorbitan monolaurate (E 432), polyoxyethylene sorbitan monooleate (E 433), polyoxyethylene sorbitan monopalmitate (E 434), polyoxyethylene sorbitan monostearate (E 435) and polyoxyethylene sorbitan tristearate (E 436) as food additives. EFSA Journal. 2015;13(7):4152, 74.
- Gupta A., Eral H. B., Hatton T. A., Doyle P. S. Nano emulsions formation properties and applications. Soft Matter. 2016;12:2826–41.
CrossRef - Smith B. T. (ed) Remington Education Physical Pharmacy. London Pharmaceutical Press. 2015;151.
- ICI Americas (ed): The HLB System A Time-saving Guide to Emulsifier Selection. Wilmington ICI Americas Inc. 1984;1–22.
- Goh P. S., Ng M. H., Choo Y. M., Boyce A. N., Chuah C. H. Production of nanoemulsions from palm-based tocotrienol rich fraction by microfluidization. Molecules. 2015;20:19936–46.
CrossRef - Gupta S., Kesarla R., Omri A. Formulation strategies to improve the bio availability of poorly absorbed drugs with special emphasis on selfemulsifying systems. International Scholarly Research Notices Pharmaceutics. 2013; 848043. DOI: 10.1155/2013/848043.
CrossRef - de Villiers M. M., Aramwit P., Kwon G. S (eds) Nanotechnology in Drug Delivery. New York: Springer. 2009;463.
- Auernhammer G., Butt H., Vollmer D. (eds) Surface and Interfacial Forces – From Fundamentals to Applications. Dordrecth: Springer 1998;124.
CrossRef - Tran T., Xi X., Rades T., Mullertz A. Formulation and characterization of self-nano emulsifying drug delivery systems containing monoacyl phosphatidylcholine. International Journal of Pharmaceutics. 2016;502(151–60):39.
- Marrufo T., Nazzaro F., Mancini E., Fratianni F., Coppola R., de Martino L., Agostinho A. B., de Feo V. Chemical composition and biological activity of the essential oil from leaves of Moringa oleifera Lam. Cultivated in Mozambique. Molecules. 2013;18:10989-11000.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





