How to Cite | Publication History | PlumX Article Matrix
Genotoxic Effect of Heavy Metals Cr, Cu, Pb and Zn Using Allium Cepa L.
M. N. Abubacker1 and C. Sathya2
1Department of Biotechnology, National College, Tiruchirappalli 620 001, Tamil Nadu, India.
2Department of Botany, Seethalakshmi Ramaswami College, Tiruchirapalli 620 002, Tamil Nadu, India.
DOI : http://dx.doi.org/10.13005/bbra/2559
ABSTRACT: Angiosperms are recognized as appropriate genetic models to detect heavy metal based environmental mutagens and are used in monitoring studies. Allium cepa (onion) has been used to evaluate DNA damages like chromosome aberrations and abnormalities in the mitotic cycle. The aim of the present study is to analyze the cytotoxic effects of chromium, copper, lead and zinc in A. cepa root tip squash mitotic cell divisions. The root tips were treated with three concentrations, viz. 5, 10 and 20 mg/100 ml of chromium, copper, lead and zinc at room temperature for 24 h. Mitotic indices and chromosomal abnormalities were calculated. It was observed that these heavy metals induced different types of chromosomal abnormalities comprising of Chromosome break, Chromosome bridge, C-mitosis, Vagrant, Delayed Anaphase and Vagrant, Chromosome Loss, Polyploidy and Chromosome Bridge, Chromosome Loss and Loculated Nucles, Stickiness, Multipolarity and Polyploid prophase along with the increasing doses. The effect of chromium and lead at 20 mg/100 ml concentration was found to be more toxic rather than copper and zinc to the root meristem of A. cepa. The ranking of cytotoxic potentials was in the descending order: lead > chromium > copper > zinc.
KEYWORDS: Allium cepa; Chromosomal Abnormalities; Heavy Metals; Mitotic Index
Download this article as:| Copy the following to cite this article: Abubacker M. N, Sathya C. Genotoxic Effect of Heavy Metals Cr, Cu, Pb and Zn Using Allium Cepa L. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Abubacker M. N, Sathya C. Genotoxic Effect of Heavy Metals Cr, Cu, Pb and Zn Using Allium Cepa L. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27483 |
Introduction
Discharge of hazardous chemicals into the environment is increasing which affected the balance of ecosystems. The heavy metals and other pollutants in water bodies and agricultural soils have led to bioaccumulation of metals in crops and accumulated in different parts of crops.1 The higher levels of heavy metals in plants suppress the metabolism and translocation of reserve food materials to the growing regions and their subsequent utilization. Heavy metals such as cadmium, chromium, iron, lead and iron cause carcinogenic in human beings and epidemiological studies on genotoxic effects of these heavy metals are reported in many biological systems.2-5 Toxicity of heavy metals, is a widespread global problem. Excess heavy metals stress in plant causes oxidative damage.6,7 Chromium and lead are unique heavy metals occur in environment in water bodies and agricultural soils.8 Chromium occur in several oxidation states as trivalent and hexavalent states in environment. Plants do not have specific mechanisms for chromium uptake and transport9. There are some non-essential metals like lead have unknown biological or physiological function.10 However hyper accumulation of toxic heavy metal ions by plants dependent on physiological mechanisms like higher rates of uptake, efficient translocation and deposition in tissue systems especially to the growing region.11
The accumulation of heavy metals cause damages, alterations to the genetic material occurring over a cell cycle.12 To test this Allium cepa root tip squash technique was conducted and it is a quick and relevant biological test for heavy metal interaction in environment and its genetic risk assessment. The test is based on the assessment of genotoxic effect of heavy metals. Chromium (Potassium dichromatic, Merck), copper (Copper-II – Sulphate, Himedia), lead (lead acetate, Merck) and zinc (zinc sulphate, Himedia) using A. cepa by recording mitotic activity (Mitotic index) and mitotic abnormalities in meristematic root tip cells.
Material and Methods
Collection of Plant Materials
The A. cepa test consists in obtaining onion bulbs cultivated without the application of herbicides, fungicides or chemical fertilizers from an agricultural field where manure alone used for cultivation. The bulbs obtained were placed initially in 50 ml culture tubes containing distilled water for 3 to 4 days for the emerging of roots. After this period the bulbs are transferred into experimental condition in which triplicate of negative control in distilled water and other positive control in 5.0, 10.0 and 20.0 mg/100 ml chromium, copper, lead and zinc all these experiment were conducted in 50 ml culture tubes for 48 h of exposure in the positive control solution.
Genotoxic Analysis
Then the rootlets were collected and immediately fixed in ethanol: acetic acid (3:1) also for 24 h. Afterwards, the rootlets were removed from the fixing solution and transferred to 70% ethanol and stored at 4°C for further experimental work. In the next stage, slides are prepared the root tips were hydrolysed in 1 N HCl for one minute for softening of tissues and then stained with 4% acetocarmine with gentle heat and the slides were prepared by using squash technique for genotoxic study, observation was done at 1000X oil immersion in light microscope (OLYMPUS CX21i LED, Camera (MagCam DC 10, 10 MP, 1/2.3” CMOS SENSOR, Software MAGVISION). The cell divisions were analyzed 400 cells per bulb total of 1200 cells per treatment and the mitotic index was calculated.13
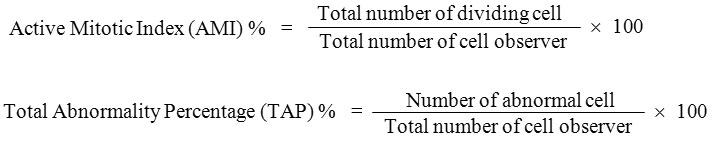
Results and Discucssion
Data of mitotic index and chromosomal abnormalities were presented in Tables 1 to 4. The statistical analysis was carried out by using Statistical Package SPSS16 version, One-way ANOVA, Post Hoc = Tukey Alpha, significant at 0.01 level. The Active Mitotic Index (AMI) characterized by the total number of dividing cells in cell cycle has been used as a parameter to assess the cyto/genotoxicity of metals. In the present study heavy metals exposed to root meristematic cells of A. cepa at different concentration have shown decrease in the mitotic index as the concentration factor is higher. The AMI for chromium was 36.66 ± 1.44, 46.66 ± 1.44, 43.33 ± 1.44 for 20 mg/100 ml, 10 mg/100 ml and 5 mg/100 ml concentrations respectively as against control 55.33 ± 1.44. For copper it was 46.66 ± 1.44, 55.83 ± 1.44, 49.66 ± 0.57 for the same concentrations as against control 60.0 ± 0.00. For lead 32.50 ± 0.00, 38.50 ± 1.32, 49.66 ± 0.57 as against control 55.00 ± 0.00 and for zinc 50.00 ± 0.00, 51.66 ± 1.44, 56.66 ± 1.44 and 60.00 ± 0.00 for control. The decrease in mitotic index with the increase in the concentration factors agree with other reports.14,15 The intensity of mitotic index was in the descending order of lead > chromium > copper > zinc. The exposure to heavy metals prevented plant cells entering cell division phases leads in a decrease in the mitotic index. The primary action of heavy metal on the mitotic spindle promoted spindle related chromosomal abnormalities during cell division.16 The decreased mitotic index treated with metal stress is due to disturbances in the cell cycle or chromatin disfunction induced by metal-DNA interaction which leads to significant reduction of mitotic index as reported in this study.17
Table 1: Mitotic index and chromosomal abnormalities in root meristematic cells of Allium cepa L. at different concentration of Chromium
| Treatment | Total no. of cells examined | No. of dividing cells | Active mitotic index
(AMI) % |
Chromosomal Abnormalities | Total no. of cells shows abnormalities | Total abnormalities
(TAP) % |
|||||||
| PP | C-M | S | CBr | MM | MA | CB | V | ||||||
| Control (deionised water) | 400 | 220 | *55.33 ± 1.44 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 2 | 6 | *1.33 ± 0.28 |
| Chromium 5 mg/100 ml | 400 | 190 | *46.66 ± 1.44 | 2 | 0 | 2 | 2 | 4 | 2 | 3 | 2 | 17 | *4.16 ± 0.14 |
| Chromium 10 mg/100 ml | 400 | 170 | *43.33 ± 1.44 | 4 | 3 | 6 | 3 | 4 | 3 | 6 | 3 | 32 | *7.66 ± 0.28 |
| Chromium 20 mg/100 ml | 400 | 140 | *36.66 ± 1.44 | 6 | 6 | 12 | 6 | 8 | 8 | 12 | 4 | 62 | *14.83 ± 0.57 |
Note:
PP: Polyploid Prophase, C-M: C-Mitosis, S: Stickness, CBr: Chromosome Bridge and break, MM: Multipolar Metaphase,
MA: Multipolar Anaphase, CB: Chromosome Break, V: Vagrant,
*: The mean difference is significance at 0.01 levels
Table 2: Mitotic index and chromosomal abnormalities on root meristematic cells of Allium cepa L. at different concentration of Copper
| Treatment | Total no. of cells examined | No. of dividing cells | Active mitotic index
(AMI) % |
Chromosomal Abnormalities | Total no. of cells shows abnormalities | Total abnormalities
(TAP) % |
|||
| LN | CL | CB | V | ||||||
| Control (deionised water) | 400 | 240 | *60.00 ± 0.00 | 2 | 0 | 2 | 2 | 6 | *1.50 ± 0.00 |
| Chromium 5 mg/100 ml | 400 | 220 | *55.83 ± 1.44 | 4 | 0 | 4 | 4 | 12 | *2.83 ± 0.14 |
| Chromium 10 mg/100 ml | 400 | 200 | *49.66 ± 0.57 | 4 | 0 | 4 | 4 | 12 | *2.83 ± 0.14 |
| Chromium 20 mg/100 ml | 400 | 180 | *46.66 ± 1.44 | 9 | 4 | 4 | 7 | 24 | *6.50 ± 0.50 |
Note:
LN: Lobulated Nuclei, CL: Chromosome Loss, CB: Chromosomal Breakage, V: Vagrant
*: The mean difference is significance at 0.01 levels
Table 3: Mitotic index and chromosomal abnormalities on root meristematic cells of Allium cepa L. at different concentration of Lead
| Treatment | Total no. of cells examined | No. of dividing cells | Active mitotic index
(AMI) % |
Chromosomal Abnormalities | Total no. of cells shows abnormalities | Total abnormalities
(TAP) % |
|||||||
| CB | CBr | C-M | V | DA &V | CL | P & CB | CL | ||||||
| Control (deionised water) | 400 | 220 | *55.00 ± 0.00 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 6 | *1.50 ± 0.00 |
| Lead 5 mg/100 ml | 400 | 180 | *46.33 ± 1.25 | 4 | 2 | 2 | 4 | 4 | 2 | 2 | 4 | 24 | *6.16 ± 0.28 |
| Lead 10 mg/100 ml | 400 | 150 | *38.50 ± 1.32 | 6 | 4 | 2 | 6 | 4 | 4 | 4 | 4 | 34 | *8.50 ± 0.50 |
| Lead 20 mg/100 ml | 400 | 130 | *32.50 ± 0.00 | 14 | 4 | 4 | 10 | 6 | 12 | 4 | 10 | 64 | *16.25 ± 0.35 |
Note:
CB: Chromosomal Breakage, CBr: Chromosome Bridge and break, C-M: C-Mitosis, V: Vagrant, DA & V: Delayed Anaphase & Vagrant,
CL: Chromosome Loss, P & CB: Polyploidy and Chromosome Bridge, CL & LN: Chromosome Loss.
*: The mean difference is significance at 0.01 level
Table 4: Mitotic index and chromosomal abnormalities on root meristematic cells of Allium cepa L. at different concentration of Zinc
| Treatment | Total no. of cells examined | No. of dividing cells | Active mitotic index
(AMI) % |
Chromosomal Abnormalities | Total no. of cells shows abnormalities | Total abnormalities
(TAP) % |
|||
| S | LN | MP | CB | ||||||
| Control (deionised water) | 400 | 240 | *60.00 ± 0.00 | 2 | 2 | 0 | 2 | 6 | *1.33 ± 0.28 |
| Zinc 5 mg/100 ml | 400 | 220 | *56.66 ± 1.44 | 2 | 0 | 2 | 2 | 6 | *1.33 ± 0.28 |
| Zinc 10 mg/100 ml | 400 | 210 | *51.66 ± 1.44 | 2 | 2 | 4 | 2 | 10 | *2.33 ± 0.28 |
| Zinc 20 mg/100 ml | 400 | 200 | *50.00 ± 0.00 | 8 | 2 | 6 | 2 | 18 | *4.00 ± 0.70 |
Note:
S: Stickness, LN: Lobulated Nucleus, MP: Multipolarity, CB: Chromosomal Breakage
*: The mean difference is significance at 0.01 levels
In the present study the genotoxic effects of chromium, copper, lead and zinc resulted in many abnormalities with reference to tolerance to heavy metal stress on root meristematic cells of Allium cepa L. Polyploid prophase, C-Mitosis, stickiness, Chromosome bridge and break, multipolar metaphase, multipolar anaphase, chromosome break and vagrant was recorded for chromium mediated stress. Copper recorded loculated nucleus, chromosome loss, chromosome break and vagrant (Fig.1). For lead chromosome break, chromosome bridge, C-mitosis, vagrant, delayed anaphase and vagrant, chromosome loss, polyploid and chromosome bridge, chromosome loss and loculated nucleus was recorded. For zinc stickiness, loculated nucleus, multipolarity and chromosome break abnormalities were resulted (Fig. 2).
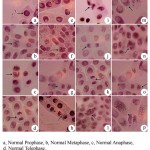 |
Figure 1: Gentoxic effect of heavy metals Chromium and Copper using Allium cepa L (onion) test (x1000)
|
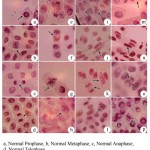 |
Figure 2: Genotoxic effect of heavy metals Lead and Zinc using Allium cepa L (onion) test (x1000)
|
The total abnormality percentage (TAP) for chromium revealed 14.83 ± 0.57 for 20 mg/100 ml, 7.66 ± 0.28 for 10 mg / 100 ml and 4.16 ± 0.14 for 5 mg/100 ml concentrations against control 1.33 ± 0.28. For copper 6.50 ± 0.50, 2.83 ± 0.14, 2.83 ± 0.14 for 20 mg, 10 mg and 5 mg/100 ml concentration respectively as against the control 1.50 ± 0.00. For lead 16.25 ± 0.35, 8.50 ± 0.50, 6.16 ± 0.28 for the above mention concentrations as against the control 1.50 ± 0.00. For zinc 4.00 ± 0.70, 2.33 ± 0.28, 1.33 ± 0.28 for the same concentrations as against the control 1.33 ± 0.28. The most frequent chromosome aberration for chromium is stickiness and chromosome break, in the case of copper loculated nucleus and vagrant, for lead chromosome break, vagrant chromosome loss and loculated nuclei, for zinc stickiness and multipolarity. The chromosome break induced by several factors, such as DNA breaks, inhibition of DNA synthesis and replication of altered DNA result from anengeric effects.18 Sticky chromosome represent poisoned chromosome with sticky surface and probably lead to cell death.16,19 The occurrence of C-Mitosis types of chromosome abnormalities are due to the loss of microtubules of the spindle fibres.13 Chromosomal losses are due to the elimination of amplified genetic material.20 The presence of Lobulated nuclei indicate a cell death process, since these abnormalities are related to nuclear abnormalities.21
Conclusion
The present study provide additional and valuable research information about the toxic effects of heavy metals by evaluating mitotic index and chromosomal abnormalities in the meristematic root cells of Allium cepa L. The result should be considered a warning of risk the environment, biota and human health may incur by natural and anthropogenic heavy metal discharge in water bodies of the environment. The Allium cepa bioassays is an integral tool in quality monitoring of water bodies.
References
- Nagajyoti P., Lee K. D and Sreekanth T. V. Heavy metals occurrence and toxicity for plants. Environ. Chem. Lett. 2010;8:199-216.
CrossRef - Sjk L., Acar O and Aaki C. Genotoxic effects of industrial waste water on Allium cepa L. Afr. J. Biotechnol. 2009;8:1919-1923.
- Samuel O. B., Osulala F and Odeigah P. G. C. Cytogenotoxicity evaluation of two industrial effluents using Allium cepa assay. Afr. J. Biotechnol. 2010;4:21-27.
- Olorunfemi D. I., Okoloko G. E., Bakare A. A and Akinboro A. Cytotoxic and genotoxic effects of cassava effluents using the Allium cepa test. Research Journal of Mutageneis. 2011;1:1-9.
CrossRef - Sharma S and Vig A. P. Genotoxicity of Atrazine, avanoxan, diuron, quisolotor-p-ethyl herbicides using Allium cepa root chromosomal aberration assay. Terrestrial and Aquatic Environmental Toxicology. 2012;6:90-95.
- Leme D. M and Marin-Morales M. A. Allium cepa test in environmental monitoring: A review in its application. Mut. Res. Rev. 2009;682:71-81.
CrossRef - Ganesan A and Panneerselvam N. Analysis of Ni induced genotoxicity in root meristem of Allium cepa. Inter. J. Biological Technol. 2013;4:19-22.
- Chandran S., Niranjana V and Benny J. Accumulation of heavy metals in waste water irrigated crops in Madurai, India. J. Environ. Res. Develop. 2012;6:432-438.
- Ambuj P. Arsenic contamination of ground water in South and East Asia. J. Environ. Res. Develop. 2012;7:70-74.
- Subhashini V and Swamy A. V. V. S. Phytoremediation of Pb and Ni contaminated soils using Catharanthus rosens L. Universal Journal of Environmental Research and Technology. 2013;3:465-472.
- Lasat M. M., Pence N. S., Curvin D. F., Ebbs S. D and Kochian I. V. Molecular physiology of the Zn transport in the hyper accumulation Thlaspi caerulescens. J. Exp. Bot. 2005;51:71-79.
CrossRef
- El-Shahaby A. O., Migid H. M. A., Soliman M. I and Mashaly I. A. Genotoxicity screening of industrial wastewater using the Allium cepa chromosome aberration assay. Pakistan Journal of Biological Sciences. 2003;6:23-28.
CrossRef
- Kumar G and Srivastava A. Comparative genotoxicity of herbicide ingredients glyphosate and atrazine on root meristem of buckwheat (Fagopyrum esculentum Moench). Jordan Journal of Biological Sciences. 2015;8:221-226.
CrossRef
- Panda S. K., Mahapatra S and Patra H. K. Chromium toxicity and water stress simulation effects in intact senescing leaves of greengram. In: Panda S. K. (ed.), Advance in Stress Physiology in Plants. Scientific Publisher, India. 2002;129-136.
- Kwankura W., Sengsni S., Muangphra P and Euawong N. Screening of plants sensitive to heavy metals using cytotoxic and genotoxic biomarkers. Kasetsart J. Natural Science. 2012;46:10-23.
- Singh P. Toxic effect of chromium on genotoxicity and cytotoxicity by use of Allium cepa L. International Journal of Research in Engineering and Applied Sciences. 2015;5:1-10.
- Kopliku D and Mesi A. Assessment of cytotoxic and genotoxic potency of Cr(VI) – Doped river water of Nen-Shkodra Lowland, Albania, on Allium cepa L. Journal of Environmental Research and Development. 2013;7:1322-1332.
- Walker C. H., Sibly R. M., Hopkin S. P and Peakall D. B. (eds.), Principal of Ecotoxicology. 4th ed., CRC Press, Florida, USA. 2012;978.
- Morais D. L and Aparecida M. Marin-Morales. Allium cepa test in environmental monitoring: A review on its application. Mutation Research. 2009;682:71-81.
CrossRef
- Leme D. M., Angelis D. F. Marin-Morales, Action mechanisms of petroleum hydrocarbons present in waters impacted by an oil spill on the genetic material of Allium cepa root cells. Aquat. Toxicol. 2008;88:214-219.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





