How to Cite | Publication History | PlumX Article Matrix
Alok Ranjan1,2, Kumari Archana2 and Sanjay Ranjan3
1CSIR-National Botanical Research institute, Lucknow,Uttar Pradesh-226015, India.
2Umea University, Umea Sweden; 901 83.
3Present address: Spectra Agritec, New Delhi – 110008 India.
Corresponding Author E-mail: alok_ranjan84@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2520
ABSTRACT: The cyclophilins genes are induced by abiotic stresses, yet their detailed function in drought and salinity remain largely unclear and need to be elaborately validated.Expression of cyclophilin was drastically induced under droughtconditions in Gossypiumherbaceum L. suggesting its stress-responsive function. In an attempt to characterize the role of G.herbacuemcyclophilingene GhCYP1, we overexpressed the GhCYP1 in tobaccousing Agrobacteriummediated transformationand explored its possible involvement in drought and salt stress tolerance.The transgenic plantsover expressing GhCYP1 exhibited tolerance against drought stress as evidenced by leaf disc assay, estimation of chlorophylland proline content along with various physiological parameters such as stomatal conductance, rate of photosynthesis and water use efficiency.The drought stressed transgenic tobaccoplants exhibited higher proline content in leaf ( 1.84 µ mol-g fw) and root (2.02µ mol-g fw ),while a reverse trend was observed in the drought stressed wild type plants, implicating the involvement of GhCYP1 in the maintenance of physiological homeostasis. Thedetail physiological, biochemical and molecular analysis results demonstrate the implicit role of GhCYP1 in conferring multiple abiotic stress tolerance at whole-plant level.
KEYWORDS: Gossypiumherbaceum; cyclophilin; GhCYP gene; abiotic stress; proline; WUE; mannitol and NaCl stress
Download this article as:| Copy the following to cite this article: Ranjan A, Archana K, Ranjan S. Gossypium Herbaceum Ghcyp1 Regulates Water-Use Efficiency and Drought Tolerance by Modulating Stomatal Activity and Photosynthesis in Transgenic Tobacco. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Ranjan A, Archana K, Ranjan S. Gossypium Herbaceum Ghcyp1 Regulates Water-Use Efficiency and Drought Tolerance by Modulating Stomatal Activity and Photosynthesis in Transgenic Tobacco. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=26668 |
Introduction
Cotton is important fiber-yielding crop plant grown worldwide. Abiotic stresses such as drought, salinity, heat, mineral deficiency, hot climate have adverse effects on the plant growth and its total fiber yield (fiber quality and its length). One of the fundamental properties of a plant to survive under stressed environment is its adaptive mechanisms where a gene must be inducible in response toa stress (Bray 1997).To understand drought induced regulatory mechanism in cotton, in our previous work we identified several stress related genes induced by water deficit condition (Ranjan et al. 2012a; Trivedi et al. 2012). One such gene is cyclophilin, also known as peptidyl-prolyl isomerases (PPIaes: EC 5.2.1.8).The peptidyl-prolyl isomeraseshasability to catalyze the inter-conversion of cis and trans isomers of proline. The physiological function of cyclophilinPPIase has been described as a chaperone or foldase(Gothel and Marahiel 1999), which helps in the folding of some proteins by rearrangements of disulfide bonds by isomerization of peptide bonds by PPIases(Galat and Metcalfe 1995). PPIases are present in a wide range of organisms, (Chou and Gasser 1997) and in organelles such as mitochondria (Anderson et al. 1993) and chloroplasts (Fulgosi et al. 1998). When plants are exposed to various environmental stresses, the heat-shock protein genesor chaperonsof different families are induced(Cui et al. 2017; Lee et al. 2016). Chaperons prevent protein aggregation, misfolding and also helps in proteolytic degradation of proteins (Hayes and Dice 1996; Mainali et al. 2014). Cyclophilins are also known to have role in diverse signaling pathways, including mitochondrial apoptosis (Leung and Halestrap 2008), RNA splicing (Teigelkamp et al. 1998) and adaptive immunity (Anderson et al. 1993). Furthermore, cyclophilin expression gets induced by both biotic and abiotic stresses including HgCl2,salicylic acid and salt stress, (Lee et al. 2015; Marivet et al. 1995; Marivet et al. 1994) heat, cold shock (Scholze et al. 1999), light (Chou and Gasser 1997) and drought stress (Sharma and Singh 2003). The cyclophilins proteins are involved in various functions viz, cell signaling, protein biogenesis and trafficking, cell cycle control, abiotic and biotic responses and regulation of membrane receptors, channels and pores (Schiene-Fischer and Yu 2001).
In our previous study of gene expression analysis of two contrasting genotypes of cotton by microarray and transcriptome sequencingunder drought stress, the different drought-responsive genes were identified (Ranjan et al. 2012a; Ranjan and Sawant 2015). However, many functions of cyclophilins were reported, the physiological relevance and molecular basis of stress-responsive expression of plant cyclophilins is still largely unknown. Zhu, et al (2011) showed that GhCYP1 play roles in salt tolerance and also tolerance in pseudomonas syringe. Here we present a physiological characterization of GhCYP1 for its possible role in water use efficiency and drought tolerence in transgenic tobacco. To address these questions, in the present study, we overexpressed the cotton GhCYP1 gene, which encodes cyclophilin,in transgenic tobacco plants. We found that the transgenic plants exhibited better growth performance under salt or drought stress. The transgenic plants showed gradual decline in photosynthesis, stomatal conductance, and rate of transpiration thus has better WUE as compared to wild type plants under water stress condition.These data suggest that GhCYP1plays an important role against stress tolerance, and might beuseful in molecular breeding to improve crop stresstolerance.
Materials and Methods
Isolation and Amplification of GhCYP1 from Cotton
The tobacco plants were grown in glass house at the CSIR-National Botanical Research Institute, Lucknow, India at 28oC, relative humidity of 50-60%, 16 h light/8 h dark photoperiod.Total RNA from leaf tissues of one month old plants (glass house grown) of G.herbaceumwere extracted using Spectrum plant total RNA Kit (Sigma-Aldrich, USA). After DNaseI treatment (Ambion), total RNA was used for first-strand cDNA synthesis using first strand synthesis kit (Invitrogen).cDNA was used as a template for amplification of full-length GhCYP1 coding sequence using Pfu DNA polymerase and primer 5’- CCATGGATGGCCTCAAATCCCAAG-3’(forward) 5’ GCTAGCCTAAGAGAGCTGTCCGCAGTC-3’ (reverse). The PCR reaction conditions were as follows: 1 cycle of 5 min at 94oC followed by 30 cycles at 94oC for 1 min, 62oC for 30sec, 72oC for 1 min with a final extension at 72oC for 5 min. The amplified PCR products were analyzed by electrophoresis on 0.8% agarose gel. Amplified PCR product of 522bp was ligated to EcoRVdigested pBluescriptSK+ vector and transformed into Escherichia coli cells. Plasmids from recombinant clones were isolated and sequenced independently with T7 and T3 promoters using automated DNA sequencer.The nucleotide and amino acid sequences were analysed by using BLAST (NCBI) and ExPASy tools. Based on sequence analysis results, it was designated as Gossypiumherbaceum CYP1 gene (GhCYP1).GenBank accession number: GQ292530.1(Zhu et al. 2011)
Cloning of Plant Expression Vector and Generation of Transgenic Plants
The full length GhCYP1 coding region (35S:GhCYP: PolyA) was cloned into NcoI and NheI sites of pCAMBIA 1301 plasmid in the sense orientation with hptII (hygromycin) expression units. The pCAMBIA 1301 and GhCYP constructs were transformed into Agrobacterium tumefaciensstrain (LBA4404) by electroporation. Agrobacterium-mediated transformation was performed using leaf explants of Nicotianatabacumas described earlier by (Riggs and Bates 1986). Transformed tobacco explants were grown on selection medium and putative T0generation plants were then shifted to glass house for seed setting. Seeds were harvested from T0 transformed plants, and plated on hygromycin (100 mgmL-1) selection medium to identify hygromycin-resistant T1 transgenic plants.Their transgenic nature was confirmed by PCR analysis using gene-specific (GhCYP1)forward primers 5’-CCATGGATGGCCTCAAATCCCAAG-3’ and reverse primer 5’-GCTAGCCTAAGAGAGCTGTCCGCAGTC-3’and CaMV35S primers (5′ATAAGAATGGCGGCCGCAAGCTT-3′ and 5′-CTAGTCTAGAAGCTTGGATCTTGTAG-3′). PCR products were analysed on 0.8% (w/v) agarose gel. Seeds of promising T1 transgenic plants were further grown on selection medium for the selection of T2 generation of transgenic plants.The expression of GhCYP1in the three T2 lines were investigated by reverse transcription–PCR (RT–PCR) analysis (data not shown). The result showed that GhCYP1 mRNA was detected in all three transgenic lines but not in the wild type (WT).The line which showed the higher expression of GhCYP1were considered for further studies.
Evaluation of Transgenic Plants for Abiotic Stress Tolerance
Seeds of the wild type and transgenic tobacco plants were germinated in soil (mixture of 1 vermiculite: 1 perlite: 1 soilrite) in a glass house, at 28oC, relative humidity of 50-60%, 16 h light/8 h dark photoperiod and a photosynthetically active radiation of 900 µmol m-2s-1 The one month old plants of approximately equal size were considered for drought stress experiments. Drought stress was given to the plants by withholding water till the wilting and drooping effect on plants leaves became apparent and soil moisture reaches below 30%. The Wet sensor machine HH2 was used to calculate the soil moisture content to determine the onset of drought. The physiological parameters were analyzed from initial day (0 day) of water withholding andupto the 17 days till drooping effect on plant leaves were most prominent. All physiological measurements were carried out on the first fully exposed leaves of wild-type and transgenic plants between 8hr to 10 hr (to avoid the involvement of daily photoinhibition). The biochemical analysis was carried out from twoweeksstressed and control plants with leaves and root sample of wild-type and T2 generation oftransgenic plants.
Assessment of Osmoticstress Tolerance of Transgenic Tobacco Plants Harbouringghcyp1 Genes
To assess the osmotic stress tolerance of transgenic plants, uniform leaf discsof 1cm2were excised from one month old transgenic and wild-type plants. The leaf discs were kept onpetriplates containing semisolid MS mediawith 2%, 4% and 6% mannitol. The MS media without mannitolwas also taken to serve as control. The seeds of wild type and transgenic plants were sterilized with 0.05% HgCl2 and thoroughly rinsed by autoclaved milliQ water in aseptic condition. The sterilized seeds were kept on NaCl (0,100 and 150 mM) and mannitol (0, 2,4 and 6%) containing MS plates. The plates were vertically placed to measure the growth androot length of two week grown seedlings under osmotic and salt stress. For the assessment of osmotic and salt stress test, five transgenic tobacco plants were considered and the experiments were repeated three times.
Analysis and Measurement of Different Physiological Parameters
The different physiological processes such as, leaf gas exchange, net photosynthetic rates (A), stomatal conductance (gs) and transpiration rate (E) was measured with an LI-6400 portable photosynthesis system (Li-Cor, Lincoln, NE) with red and blue LED light sources. All measurements were performed on the first fully exposed leaves of one month old plants of wild type and transgenic plants between 8hr to 10 hr (to avoid the involvement of daily photoinhibition). The control samples were watered at alternate days while drought stress was imposed by withholding water for 17 days.
Physiological Experiment Design
The photosynthetic photon flux density (PPFD), 400 µmol m-2s-1, leaf temperature 25oC,leaf-air vapour pressure deficit (VPD) <2.0 KPa, and the levels of CO2 inside the leaf cuvette was maintained at 400 µmol mol-1,during gas exchange and fluorescence measurements. The chlorophyll fluorescence parameter Fv/Fm (maximum photochemical efficiency of photosystem II in the dark-adapted state) was measured as described previously by (Bolhar-Nordenkampf et al. 1989) with a portable chlorophyll fluorometer. The photosynthetic efficiency and fluorescence studies were made on separate days at same hour as mentioned above and physiologically mature leaves (6-8th) were selected for all photosynthetic measurements, using the Walz, GFS 3000 system with the LED-Array/PAM-Fluorometer 3055-FL” module head attached to the cuvette.The water use efficiency (WUE) was calculated as the ratio of (A) to (E) .Dark respiration (R) was measured under similar microclimatic conditions after dark-adaptation of leaf for more than 30 min. The relative water content (RWC) was made at predawn on single, fully expanded leaves (third and fourth leaves from the terminal bud of a twig) immediately after excision. TheRelative Water Content (RWC) of leaf was calculated as 100× (fresh weight – dry weight) / (turgid weight – dry weight).All the data presented were the means ± SD from three independent experiments (n = 12), and all the conditions were tested with significant differences at p<0.05 level.
Chlorophyll Estimation
Estimation of Chlorophyll was done according to (Arnon 1949) method. The 100 mg leaf samples or leaf discs with three replicates for each treatment was ground in 80% acetone (10 ml), and centrifuged at 10000×g for 10 min. Absorbance of supernatant was measured at 645 nm and 663 nm using spectrophotometer. The concentration of extracted “chlorophyll a” and “chlorophyll b” in aqueous phase (80% acetone) was determined with a Beckman spectrophotometer at 663 and 645 nm absorption respectively. The data were presented in mean of three biological replicates sample.
Measurement of Proline Content
The proline content in the leaf and root tissue was estimated according to the method suggested by (Bates et al. 1973). The leaf sample of 500 mg was homogenized in 10 ml of 3% sulphosalicylic acid. The filtered homogenate was used for the estimation of proline. A 2 ml of filtrate was taken in a test tube to which equal volumeof ninhydrin reagent (2.5 g of ninhydrin dissolved in 40 ml of 6 M orthophosphoric acid) and 60 ml of glacial acetic acid were added. The test tubes containing the mixture were placed in boiling water bath set at 100oC for an hour followed by transferring to 4oC and further 4 ml of toluene was added. Thesolution wastransferred to separating funnel and shaken vigorously and allowed to settle for few minutes for the separation of two immiscible layers. The lower layer was discarded and upper solvent layer of toluene was pooled into a test tube. The colorimetric estimation was carried out with spectrophotometer at 520 nm. A blank was maintained with all the reactants except the leaf extract.
Results
Generation and Overexpression of CYP1 Gene in Transgenic Plants
The GhCYP1 coding sequence was cloned under the control of the cauliflower mosaic virus 35S promoterusing pCambia 1301 vector. The constructwas introduced in Nicotianatabacum through Agrobacteriummediated transformationto obtain transgenic tobacco plants. To confirm the integration of the CYP1 gene, we performed PCR using genomic DNA as template. GhCYP1specific primers were used to amplify a 522 bpfragment using genomic DNA from transgenic plants. The nucleotides sequences of amplified PCR product were also analysed by using Sanger sequencing to to confirm the GhCYP1 fragment. The GhCYP1 fragment was detected in transgenic plants; while no amplification was observed in the DNA from wild type (WT)plants. Further, the expression of CYP1 gene in tobaccoplants was analysed by RT-PCR and those plants showed high expression were selected (data not shown) for further analysis.
Increased Osmotictolerance Inghcyp1transgenic Plants
The detached leaf disc assay along with its chlorophyll estimationrevealed thatupto 4% of mannitol concentration leaf disc of transgenic plants showed healthy survival but wild type leaf discbecome yellow and shrunken (radius decreased) with relative chlorophyll content being 67% and 43% of transgenic and wild type plants, respectively (Fig.1a and 1b).Further leaf discs from wild type at 6% mannitol solution became significantly bleached and chlorophyll content was markedly reduced to 28% while leaf discs of transgenic plants had significantly higher relative chlorophyll content of 62%(Fig.2b). Leaf disc from wild type showed pronounced chlorophyll bleaching symptomswith lower chlorophyll contenthence more susceptible to osmotic stress than the transgenic plant.
Seeds of Transgenic Plants Showed Better Germination Under Osmotic Stress
The seed germination test indicated that over-expression of GhCYP elevated the sensitivity of transgenic plants to osmotic stress, whereas no difference was found in seed germination between the wild type and transgenic plants under control condition (0% mannitol) (Fig. 1c). At 4% and 6% mannitol, few transgenic seeds (T) germinated and survived, while those of the wild type (WT)showed negligible survivability and germination hence showed high susceptibility for the survival in the stressed environment (Fig.1c).The seeds of transgenic plants were able to germinatein the presence of 4% mannitol, while seeds of wildtype plants failed to germinate.The more number of seedsfromtransgenic plants were germinated at higher percentage of mannitol which revealed that the CYP gene imparted the tolerance to the seed required for the germination in the stress condition.
Assessment of Transgenic Seedling and Root Growth Under Stress Condition
The responseof early seedling growth and root length of transgenic and wild types plants were examined at different salt(0mM, 100mM and 150mMNaCl) and mannitolconcentration (0%,2%, and 4%). Seedling of transgenic plants showed healthier growth on 100and 150mM NaCl whereas in case of wildtype seedling growth and root length was strongly inhibited and plants became stunted (Fig. 2a). Wild type had curved and broken roots at 150mM NaCl while transgenic plants survived through the stress with relatively longer and healthy primary root.The similar pattern was also observed in the length of root development under osmotic stresswith transgenic seedlings showing longer primary root length while wild type exhibited relatively stunted root growth at 2% mannitol. The severity of stress was more apparent on wild type at 4% mannitol (Fig. 2b). Transgenic plants showed longer root length at 150mM NaCl and at 4% mannitol as compared to wildtype (Fig. 2a and 2b).The transgenic plants survived and performed better under water deficit and salinity conditions, while wildtype plants failed to withstand stress at 100mMNaCl and 4% mannitol.
Physiological Parameters Measurements Under Drought Stress
The rate of photosynthesis, ETR,qN and NPQ were measured under control conditions and no significant differences were observed between wildtype and transgenic plants. However, during water deficit condition (after 7 days) the wildtype plants showed a marked reduction in ETR and qN and an increase in NPQ (Fig. 3a,3b and 3d). The transgenic plants displayed substantially higher thermal dissipation (NPQ) under control and drought condition as compared to wildtype plants (Fig.3d). Further we examined several representative physiological parameters that control plant vigor such as transpiration rate, a trait generally associated with the rates of water consumption and transport in the plant; photosynthesis rate, a trait positively correlated with plant vitality and biomass production; photochemical quantum efficiency (measured as the chlorophyll fluorescence parameter Fv/Fm [maximum photochemical efficiency of photosystem II in the dark-adapted state]), a trait positively correlated with the organization and vitality of photosystem II; and water use efficiency, a parameter indicating drought tolerance ability to plants. Results of gas exchange parameters under control condition showed marginal differences in the rate of photosynthesis (A) and stomatal conductance (gs) in wildtype and transgenic plantsshowed slightly higher A and gs (Fig.4a and 4d). However, after water restricted condition for 10 days, transgenic plants showed sharp decrease in rate of photosynthesis (A), stomatal conductance (gs) and rate of transpiration (E) while in wildtype plants significant differences were not observed. The rate of transpiration was observed higher in wildtype plants than that in transgenic plant under control condition and further increased after water restricted condition (Fig.4a,4b and 4d). The rate of respiration was lower in transgenic plants as compared to the wildtype (Fig.4c), and slightly decreased after drought stress while in wildtype plants, respiration rate was continuously increased which was almost 2 fold after 10 days of drought. After 7 days of drought stress treatment transgenic plants showed substantial differences in photochemical quantum efficiency under drought condition as compared to wild type (Fig.4d). The WUE was higher in transgenic plants as compared to wildtype plants under irrigated condition and decreased under moderate drought in both plants (Fig.4f).
Chlorophyll Estimation Under Drought Stress
Leaf sample from one month old transgenic and wildtype were used for spectrophotometric estimation of chlorophyll after extraction according to method described by (Arnon 1949). Whole plants were subjected to drought stress and revealed higher chlorophyll content as compared to the wild type plants but no significant change was observed under normal watered condition in transgenic and wild type plants (Table 1).The reduced level of chlorophyll in wild type under stress condition could be attributed to the gradual degradation of the chlorophyll due to water deficit environment.
Proline Estimation Under Drought Stress
Osmoprotectants such as proline, glycine, betaine, and sugar alcoholplay major role in plant for adaptation to drought(Verbruggen and Hermans 2008).Therefore proline content was measured for assessing the role of GhCYP1 in drought tolerance. Besides higher chlorophyll content, the striking differences in proline accumulation was observed in leaf and root tissue of transgenicplants under drought condition compared to wild type. There was approximately more than 4.7 and 3.8-fold increase in the proline accumulation in leaf and root tissue respectively underdrought in the transgenic plants(Table 1). Hence, we can clearly state that overexpression of GhCYP1 can lead to accumulation of greater quantities of proline under control as well as drought condition, which helps transgenic plant to adapt better to drought.
Discussion
Cyclophilins are a family of proteins that are found inall cells of all organisms studied from prokaryotes to eukaryotesand they are structurally conserved throughout evolution (Romano et al. 2005). CYPs belong to various families (immunophilin family) are known to possess enzymatic peptidyl-prolyl isomerase activity essential for protein folding in-vivo, thereby suggesting their cardinal importance in different metabolic processes. In our earlier global gene expression studies of G.herbaceum under drought condition showed more than twenty fold higher expressions of GhCYP1 gene(Ranjan et al. 2012a), which indicates that the CYP gene play an important role in tolerance to drought in cotton (Ranjan et al. 2012a).In this study, for functional validation of the GhCYP1 gene in a heterologous host, tobacco plants were transformed with GhCYP1 to evaluate its function in abiotic stress tolerance. Transgenic tobacco plants expressing GhCYP1 were able to withstand abiotic stresses imposed by osmotic (mannitol), salt and drought stress (Fig 1a, 1c and 2a, 2b). The leaf disc of transgenic plants remains healthy and maintained its greencolourwith treatment of up to 4% mannitol concentration butwild type leaf disc become yellowish and shrunken(Fig.1a). Our results were in accordance with the earlier studies on other plant speciesharboured with different drought tolerance genes(Marivet et al. 1994; Sharma and Singh 2003).
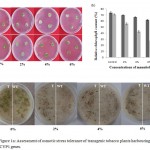 |
Figure 1a: Assessment of osmotic stress tolerance of transgenic tobacco plants harbouringGhCYP1 genes. |
Leaf discs of one month old transgenic and wild type plants were subjected to osmotic stress at 0%, 2%, 4% and 6% of mannitol, (b) Relative chlorophyll content in leaf discs from transgenic and wild type plants after treatment with different concentrations of mannitol and (c) Rate of seed germination under osmotic stress in transgenic plants. WT – wild type, T- transgenic plants.
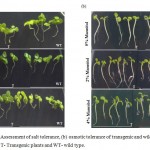 |
Figure 2a: Assessment of salt tolerance, (b) osmotic tolerance of transgenic and wild type plants (left side). T- Transgenic plants and WT- wild type. |
Among the fluorescence parameters, the maximum quantum efficiency (Fv/Fm) was most stable in well-watered plants and yielded significant contrasts between the wildtype and transgenic plants after 10 day of induced drought stress (Fig.4e). Significantly reduced depression of Fv/Fm in transgenic plants under severe drought as compared to the corresponding case of wildtype plants indicates that transgenic plants are more efficient in protecting their photosynthetic apparatus(Oukarroum et al. 2007). Transgenic plants respond to drought by gradual decline in A, gs and E and thus have better WUE (Fig.4a, 4b, 4d and 4e) as compared to wildtype plants.The gradual decrease in photosynthesis rate and stomatal conductance in transgenic plants showed its efficient water utilization properties as compared to wild type plants.However, we noticed a significant increase in NPQ in transgenic plants when subjected to drought stress as compared to wild type plants (Fig.3d). The rise in NPQ probably suggests that stomata were severely closed thus inhibiting the CO2 supply in the leaf chloroplasts. On the other hand, there was decrease in dark respiration (R) in transgenic plants with the onset of drought while R in drought stressed wild type plants was higher (Fig.4c).The reduction in ETR (Fig.3a) and qN (Fig.3b) and the increase in NPQ in transgenic plants suggest a decrease in energy transfer to the PSII core complexes and a possible increase in cyclic electron transfer during drought stress.The results on total chlorophyll content differed significantly in transgenic plants as compared to wild type plants under drought stress (Table 1). With the onset of drought stress, there was decrease in total chlorophyll content in transgenic as well as wild type plants. The lack of effects on the chlorophyll a/b ratio indicates that chlorophyll b is not much sensitive to drought than chlorophyll a (Table 1). The transgenic plants showed a higher chlorophyll a content as compared to wild type (Table 1).Concurrent with our results, in 2001, Nyachiro, et al. reported a significant decline of chlorophyll a and b caused by water deficit in Triticumaestivum cultivars(Nyachiro et al. 2001). Decreased or unchanged chlorophyll level during drought stress has been reported in other species, depending on the duration and severity of drought(Kyparissis et al. 1995). A decrease of total chlorophyll with drought stress implies a lowered capacity for light harvesting. The production of reactive oxygen species (ROS)is mainly driven when the absorption of light exceeds the capacity for photosynthetic metabolism. To avoid this, the plant must ensure that the excess energy is dissipated harmlessly thereforethe generation of ROS might be avoided by degrading the absorbing pigmentsi.e. cytochromebf complex(Loreto et al. 2004).Similarly higher content of proline in leaf and root tissue of transgenic plants under water deficit condition showed their survival (Table 1). A positive effect of increased proline content on salt stress tolerance has been observed by other researchers in transgenic plants (Zhang and Shih 2007). It is well documented that under stress conditions, many osmotically active compatiblesolutes accumulate in leaves and roots of higher plants, which lowers the osmotic potential and help to maintain the turgor pressure of cells(Ranjan et al. 2012a; Ranjan et al. 2012b; Zhang and Shih 2007).The molecular mechanism for drought tolerance by Cyclophilins not very well documented. However, GhCYP1have been found to possess PPIase and a chaperone-like activity as a result of a search for an enzyme that catalyses the slow cis±transisomerization of prolyl imide bonds in peptide chains during protein folding and protecting them from degradation and aggregation (Sharma and Singh 2003; Stewart et al. 1990). Protein folding occurs in several subcellular compartments in addition to the cytosol. Consistent with an important role for CYP in this process, members of the CYP family have been found in the endoplasmic reticulum and mitochondria. A number of predicted cytosol-localized CYPswere shown totarget the nucleus by enhancing nucleic acid interactions. Meanwhile, some CYPs were shownto be localized in the nucleus to interact with transcriptionactors, as well as RNA polymerases, to control gene expression,a property that may help plants grow successfully under various biotic and abiotic stresses(Mainali et al. 2017; Shaw 2007).Although a preliminary understanding of themechanism for induction of stress tolerance in plants through CYPswas developed, further studies are necessary to establish theirphysiological role in various stresses.Increased tolerance to salinity was also reported in transgenic tobacco, transformed with the GhCYP gene (Zhu et al. 2011). The earlier report on GhCYP, demonstrated the effective role of GhCYP gene in biotic and abiotic stress tolerance when overexpressed in tobacco(Zhu et al. 2011). Transcript analysis of GhCYP by qRT-PCR of stressed transgenic tobacco plants, using GhCYP gene specific primer showed enhanced transcript levels under drought stress as compared to weak signals in the unstressed plants, thus indicating the stress responsive nature of GhCYP gene (Fig.1a). Therefore establishing the role of GhCYP in mitigating the effects of abiotic stress by minimizing partial folding of proteins or by promoting the dissociation of protein aggregates which were also shown in microbes (Boston et al. 1996; Nitta et al. 2004). In summary, our data shed light on the role of cyclophilin gene of cotton in drought and salt stress response. These results point the way towards the use of GhCYP gene as potential candidate genes for engineering tolerance to drought and salt stress.
Table 1: Drought stress induced changes in chlorophyll (mg g −1 fresh weight) and proline (µ mol g −1 fresh weight) content.
| Sample | Chlorophyll a (mg g -1fw) | Chlorophyll b (mg g -1fw) | Total Chlorophyll (mg g -1fw) | Chlorophyll a/b | Proline
(µ mol-g fw) Leaf Root |
|
| Wild Type Control | 1.56 | 0.84 | 2.4 | 1.85 | 0.25 | 0.22 |
| Wild Type Drought(Dt-14) | 1.19 | 0.55 | 1.74 | 2.16 | 1.51 | 1.32 |
| Transgenic Control | 1.82 | 0.92 | 2.74 | 1.97 | 0.43 | 0.48 |
| Transgenic Drought(Dt-14) | 1.58 | 0.64 | 2.22 | 2.46 | 2.02 | 1.84 |
Results shown as mean±standard error (p<0.05), obtained from three replicates. The 14 days’ drought stress plants were considered for experiments
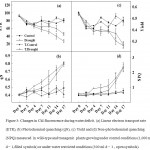 |
Figure 3: Changes in Chl fluorescence during water deficit. (a) Linear electron transport rate (ETR), (b) Photochemical quenching (qN), (c) Yield and (d) Non-photochemical quenching (NPQ) measured in wild-type and transgenic plants growing under control conditions (1,000 ml d − 1,filled symbols) or under water restricted conditions (300 ml d − 1 , open symbols). |
All measurements were performed as described in the Materials and Methods. Each data point represents the mean ± SE (n = 12). (Symbols represents -○- WT control, -●- WT-drought, -▲- T.drought, -∆- T.control. WT – wild type, T – transgenic plants.
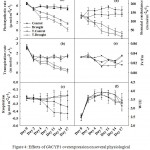 |
Figure 4: Effects of GhCYP1 overexpression on several physiological parameters of the Plants. |
(a) photosynthesis rate (A, μmol m–2 s–1), (b) transpiration rate (E, mmol m–2 s–1), (c) respiration (μmol m–2 s–1), (d) stomatal conductance (gs, mmol m–2 s–1), (e) the chlorophyll fluorescence parameter Fv/Fm and (f) water use efficiency (WUE, mmol CO2 mol H2O m–2 s–1). Measurements were performed on the first fully exposed leaves of wild type and transgenic plants.
Conclusion
The cyclophilins proteins involved in a variety of cellular functions (e.g. cell cycle, signal transduction, energy metabolism, cellular detoxification and gene regulation) indicates that these molecules could affect the molecular mechanism of water-deficit stress tolerance in transgenic tobacco plants.The transgenic tobacco plants showed higher RWC with respect to wild type plants, which facilitate active absorption of water through osmosis. It is also justified, as more proline accumulation in leaf and root under water deficit condition in transgenic plants as compared to wild type plants. The transgenic plants showed comparatively high photosynthesis rates due to high cyclic electron flow under mild stress. In wild type, cyclic electron flow initiates at low light; however, transgenic plants maintained cyclic electron flow even at severe drought and has high Y(NPQ) to dissipate light energy as heat.
Acknowledgement
The work was carried out at CSIR-National Botanical Research Institute (NBRI), Lucknow. Council of Scientific and Industrial Research, India, is gratefully acknowledged for financial support to AR and SR as Senior Research Fellow at NBRI. We thank Dr. Samir Sawant, Scientist NBRI, for providing experimental facilities for conducting all the experiments.
Author contributions
AR and SR conceived and designed the study. AR, KA and SR performed the experiments and AR drafted the manuscript. All authors read and approved the final manuscript.
References
- Bray A. Plant responses to water deficit. Trends in Plant Science. 1997;2(2)48-54.
CrossRef - Ranjan D. N., Asif M. H., Singh R., Ranjan S., Mantri S., Pandey N., Trivedi I., Rai K. M., Jena S.N., Koul B., Tuli R., Pathre U. V. ,Sawant S. V. Genome wide expression profiling of two accession of G. herbaceum L. in response to drought. BMC Genomics. 2012;13:94.
CrossRef - Trivedi A. R., Sharma Y. K., Sawant S. The Histone H1 Variant Accumulates in Response to Water Stress in the Drought Tolerant Geno type of Gossypiumherbaceum L. The Protein Journal. 2012;31(6):1-10.
CrossRef - Anderson K. S., Gallinger J. R., Frey J., Young H. A., Ortaldo J.R. A cyclophilin-related protein involved in the function of natural killer cells. Proc Natl AcadSci U S A . 1993;90(2):542-6.
CrossRef - Fulgosi A.V., Vener L. A., Herrmann R. G.,Andersson B. A novel multi-functional chloroplast protein: identification of a 40 kDaimmunophilin-like protein located in the thylakoid lumen. EMBO J. 1998;17(6):1577-87.
CrossRef - Lee S. S., Lee W. Y., Jung H. J., Park B. R., Lim H. S., Kim J. C., Ahn H. S., Cho. The OsCYP19-4 Gene Is Expressed as Multiple Alternatively Spliced Transcripts Encoding Isoforms with Distinct Cellular Localizations and PPIase Activities under Cold Stress. Int J MolSci. 2016;17(7).
- Cui H., Liu S., Ruan B., Ali R. A., Gill H., Ma Z., Zheng W. Z. A zinc finger protein, interacted with cyclophilin, affects root development via IAA pathway in rice. J Integr Plant Biol . 2017.
CrossRef - Hayes A., Dice J .F. Roles of molecular chaperones in protein degradation. J Cell Biol. 1996;132(3):255-8.
CrossRef - Mainali R.,Chapman P., Dhaubhadel S. Genome-wide analysis of Cyclophilin gene family in soybean (Glycine max). BMC Plant Biol. 2014;14:282.
CrossRef - Stewart E., Sarkar A., Wampler J. E. Occurrence and role of cis peptide bonds in protein structures. J MolBiol. 1990;214(1):253-60.
CrossRef - Leung W., Halestrap A. P. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta. 2008;1777(7-8):946-52.
CrossRef - Teigelkamp T., Achsel C., Mundt S. F., Gothel U., Cronshagen W. S., Lane M., Marahiel R. L. The 20kD protein of human [U4/U6.U5] tri-snRNPs is a novel cyclophilin that forms a complex with the U4/U6-specific 60kD and 90kD proteins. RNA. 1998;4(2):127-41.
- Marivet, M. Margis-Pinheiro, P. Frendo, G. Burkard, Bean cyclophilin gene expression during plant development and stress conditions. Plant MolBiol. 1994;26(4):1181-9.
CrossRef - Marivet P., Frendo G. B. DNA sequence analysis of a cyclophilin gene from maize: developmental expression and regulation by salicylic acid. Mol Gen Genet. 1995;247(2):222-8.
CrossRef - Lee S., Park H. J., Yoon D. H., Kim B. G., Ahn J. C., Luan S., Cho H.S. Rice cyclophilin OsCYP18-2 is translocated to the nucleus by an interaction with SKIP and enhances drought tolerance in rice and Arabidopsis Plant Cell Environ. 2015;38(10):2071-87.
CrossRef - Scholze A. P., Diettrich B., Luckner M. Cyclophilin isoforms from Digitalis lanata. Sequences and expression during embryogenesis and stress. Journal of plant physiology. 1999;155(2):212-219.
CrossRef - Sharma D., Singh P. Effect of water stress on expression of a 20 kDcyclophilin-like protein in drought susceptible and tolerant cultivars of Sorghum. Journal of Plant Biochemistry and Biotechnology. 2003;12.
- Schiene-Fischer C. Y. Receptor accessory folding helper enzymes: the functional role of peptidyl prolyl cis trans isomerases. FEBS Lett. 2001;495(1-2):1-6.
CrossRef - Ranjan S. S. Genome-wide transcriptomic comparison of cotton (Gossypiumherbaceum) leaf and root under drought stress. 3 Biotech. 2015;5(4):585-596.
- Zhu Y., Wang Y., Li K. H., Bhatti Y., Tian J. W. Overexpression of a cotton cyclophilin gene GhCyp1 in transgenic tobacco plants confers dual tolerance to salt stress and Pseudomonas syringaetabaci infection. Plant Physiology and Biochemistry. 2011;49(11):1264–1271.
CrossRef - Riggs D., Bates G. W. Stable transformation of tobacco by electroporation evidence for plasmid concatenation. Proceedings of the National Academy of Sciences. 1986;83(15):5602.
CrossRef - Bolhar-Nordenkampf R., Long S. P., Baker N. R., Oquist G., Schreiber U., Lechner E. G. Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. Functional Ecology. 1989;497-514.
CrossRef - Arnon I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris Plant Physiology . 1949;24(1):1.
CrossRef - Bates S., Waldren R. P., Teare I. D. Rapid determination of free proline for water-stress studies. Plant and soil. 1973;39(1):205-207.
CrossRef - Verbruggen C. H. Proline accumulation in plants a review. Amino acids. 2008;35(4):753-759.
CrossRef - Romano J.,Gray P., Horton S. L. Plant immunophilins functional versatility beyond protein maturation. New phytologist. 2005;166(3):753-769.
CrossRef - Oukarroum S. E., Madidi G. S., Strasser R. J. Probing the responses of barley cultivars Hordeum vulgare by chlorophyll fluorescence OLKJIP under drought stress and re-watering. Environmental and Experimental Botany. 2007;60(3):438-446.
CrossRef - Nyachiro M., Briggs K. G., Hoddinott J., Johnson-Flanagan A. M. Chlorophyll content, chlorophyll fluorescence and water deficit in spring wheat. Cereal Research Communication. 2001;29(1/2):135-142.
- Kyparissis Y. P., Manetas Y. Summer survival of leaves in a soft-leaved shrub (Phlomisfruticosa L., Labiatae) under Mediterranean field conditions: avoidance of photoinhibitory damage through decreased chlorophyll contents. Journal of experimental botany. 1995;46(12):1825-1831.
CrossRef - Loreto N. R. B., Ort D. R. Chloroplast to leaf. Photosynthetic Adaptation. 2004;231-261.
- Zhang D. S. S. Isolation of an osmotin-like protein gene from strawberry and analysis of the response of this gene to abiotic stresses. Journal of plant physiology. 2007;164(1):68-77.
CrossRef - Ranjan N., Pandey D., Lakhwani N. K., Dubey U. V., Pathre S. V. S. Comparative transcriptomic analysis of roots of contrasting Gossypiumherbaceum genotypes revealing adaptation to drought. BMC Genomics. 2012;13:680.
CrossRef - Shaw E. Peptidyl-prolyl cis trans isomerases and transcription: is there a twist in the tail? EMBO reports. 2007;8(1):40-45.
CrossRef - Mainali R., Vadivel A. K., Li X., Gijzen M., Dhaubhadel S. Soybean cyclophilin GmCYP1 interacts with an is of lavonoid regulator GmMYB 176. Sci Rep. 2017;7:39550.
CrossRef - Boston S., Viitanen P. V., Vierling E. Molecular chaperones and protein folding in plants Plant. Molecular Biology. 1996;32(1):191-222.
CrossRef - Nitta Y., Kaneko K., Kojima H.,Fukuzawa H., Kosaka H. N. Comparative analysis of the hspA mutant and wild-type Synechocystis sp. strain PCC 6803 under salt stress: evaluation of the role of hspA in salt-stress management. Archives of Microbiology. 2004;182(6):487-497.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





