How to Cite | Publication History | PlumX Article Matrix
Human Cancer Genetics, Stem Cells, and Medical Molecular Biology: An Epigrammatic Review
Atif Abdulwahab A. Oyouni
Department of Biology, Faculty of Sciences, University of Tabuk, Tabuk, Kingdom of Saudi Arabia. Corresponding Author E-mail: a.oyouni@ut.edu.sa
DOI : http://dx.doi.org/10.13005/bbra/2521
ABSTRACT: Cancer is a relatively common disease that affects millions of people worldwide. Although cancer itself has been highly researched, discovering a cure for cancer remains a challenge, primarily because the causes of this disease are not entirely understood. It can arise from mutations and epigenetic alterations that go on to activate oncogenes and inactivate tumour suppressor genes. The cells that drive cancer formation proliferate in an uncontrolled manner and originate from various pathways, which have been highlighted in this review. Briefly, cancer stem cells can arise from three different scenarios: a) a stem cell undergoes mutation, b) the progenitor cell undergoes several mutations and c) an already differentiated cell re-differentiates due to mutation to drive it back to a stem cell-like state.
KEYWORDS: Cancer; Genetics; Molecular biology Stem cell;
Download this article as:| Copy the following to cite this article: Oyouni A. A. A. Human Cancer Genetics, Stem Cells, and Medical Molecular Biology: An Epigrammatic Review. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Oyouni A. A. A. Human Cancer Genetics, Stem Cells, and Medical Molecular Biology: An Epigrammatic Review. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27710 |
Introduction
Cancer is a common illness affecting an estimated 25 million people around the world and leading to seven million deaths each year (Popat et al., 2013). Figure 1A and 1B illustrates the prevalence of several common types of cancer. This illness is characterised by the development of a subset of neoplasms; therefore, it is medically referred to as a malignant neoplasm. The term cancer covers large subset of diseases leading to unregulated cell growth. In a cancerous state, cells divide and proliferate uncontrollably and form malignant tumours, which can then spread to other parts of the body. It can spread particularly quickly through the whole body if the cells invade the lymphatic system or bloodstream. Cancer is caused by a number of complex pathways that are not entirely understood; however, it is known that smoking, diet and environmental pollutants can affect cancer development. Such factors can have direct effects on the genes, causing damage. It is estimated that 90–95% of cancer cases are caused by environmental factors, whereas only 5–10% are linked directly to genetics (Anand et al., 2008).
Cancer Genetics
Cancer is caused by changes to three types of genes: oncogenes, tumour-suppressor genes and stability genes. In contrast to other diseases, such as cystic fibrosis and muscular dystrophy, in which the mutations in one gene lead to the disorder, no single gene mutation causes cancer. A number of genes must become mutated before invasive cancer will develop.
Oncogenes
Oncogene activation often results from chromosomal translocations, which arise from gene amplification as well as indirect mutations that affect the regulation of gene product activity. For example, studies have shown that the mutation of the BRAF gene in human cancer can be due to changing a valine to a glutamine at codon 599 (Pedone et al., 2013). The activation pathway is regulated by the phosphorylation of Thr598 and Ser601 residues
882 OYOUNI., Biosci., Biotech. Res. Asia, Vol. 14(3), 881-885 (2017) under normal conditions. This finding suggests that the substitution of glutamine at codon 599 activates enzymes, even in the absence of phosphorylation signals from the adjacent Thr598 and Ser601 residues. Consequently, the activated BRAF kinase phosphorylates downstream targets and causes abnormal growth. A single somatic mutation in an oncogene can cause cells to progress and form an abnormal growth
Tumour Suppressor Genes
Tumour suppressor genes can regulate a number of different cellular activities, including DNA damage, cell cycle arrest, cell differentiation and programmed cell death (Vurusaner et al., 2012). Tumour suppressor genes vary from oncogenes in that the mutations lead to a reduction in the activity of the gene product. The reduced activity occurs due to mutations that take place in the residues that are vital for gene activity. Studies have demonstrated that damage to both the maternal and paternal alleles of a gene is required for cells to be susceptible.
A recent study was carried out to determine the effects of physical activity on DNA methylation, which went on to predict any effects on gene expression and breast cancer survival (Zeng et al., 2012). The study analysed three different genes which were associated with breast cancer survival. The researchers found that an increase in physical activity can lead to the epigenetic regulation of tumour suppressor genes and could have favourable effects on breast cancer survival rates.
There are a number of tumour suppressor genes in the human body, and their expression is dependent on the location. The areas of specific tumour suppressor genes within tumours are not fully known; however, a recent study investigated the genes expressed in colon neoplasms (Luo et al., 2013). The researchers identified high levels of methylated RET in the cancer tissue. RET is a transmembrane receptor tyrosine kinase and a
It has been identified as an oncogene in thyroid cancer and pheochromocytoma. In that study, the researchers reported that methylated RET was found in 27% of colon adenomas and 63% of colorectal cancers, and the results suggested that RET may exhibit tumour suppressive activity in colon cancer. Additionally, the methylation of RET gave rise to decreased RET expression, whereas the reinstatement of RET in the colon cancer cell lines resulted in apoptosis. From this study, the researchers were able to conclude that RET can act as a tumour suppressor gene in the colon, and that the inactivation of RET leads to the progression of colon adenomas to cancer.
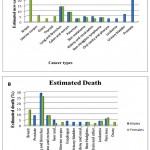 |
Figure 1: The ten leading cancer types, with estimates of new cancer cases and deaths in the USA in 2012 (Siegel et al., 2012)Click here to View figure |
DNA Repair Genes
Cancer can also be caused by damage during DNA replication. The Spo11 gene is of great interest to researchers since it has been shown to play a critical role in stimulating double stranded break (DSB) formation, which is essential for correct DNA formation (Schuldt, 2013). Spo11 is essential for DSB formation, and studies have shown that without the gene, DSB formation is interrupted, which can have serious implications due to the consequent misalignment of the DNA strands. In this way, DSBs act as potential inducers of mutations and cell death (Schuldt, 2013). For example, in Metazoa, a single DSB can induce the apoptosis of cells since the presence of a DSB can lead to the inactivation of a gene. DSBs are also significantly implicated in the development of cancer through the induction of mutations and chromosomal translocations (Deans and West, 2011). These chromosomal translocations arise from the creation of chromosomal DNA DSBs that are ligated together through the DNA repair process. Cancers of lymphoid origin often contain these chromosomal translocations, which are a consequence of malfunctioning DSB repair.
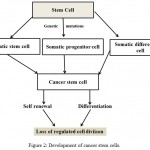 |
Figure 2: Development of cancer stem cells.
|
Stem Cells and Cancer Stem Cells
Stem cells are characterised by their ability to self-renew and their capacity to differentiate into diverse specialised cell types (Wong et al., 2013). Human stem cells include embryonic stem cells (ESCs), adult stem cells, cancer stem cells (CSCs) and induced pluripotent stem cells (iPSCs) (Yu et al., 2012). As stem cells transform to a differentiated state, self-renewal ensures the generation of undifferentiated stem cells. The differentiation of the stem cells leads to the formation of mature cell types. ESCs are of particular interest since they have the ability to differentiate into all cell types during embryonic development (Stavridis, 2013). Adult stem cells play pivotal roles in the replenishment and repair of adult tissues. In recent years, researchers have been able to re-programme somatic cells into stem cell-like cells that have the characteristics of ESCs and are referred to as iPSCs (Zhou et al., 2013; Takahashi and Yamanaka, 2013). These cells have the potential to be used in a number of therapeutic applications, particularly in the fields of tissue regeneration and engineering (Fox and Duncan, 2013; Diekman et al., 2012; Wong et al., 2013).
In addition to the development of iPSCs, a subpopulation of stem cell-like cells has recently been located in tumours called CSCs (Kesanakurti et al., 2013). These cells are distinct in that they exhibit both stem cell and cancer cell characteristics. Like normal stem cells, CSCs have the ability to self-renew and differentiate (Nguyen et al., 2012). However, they also have the ability to seed tumours into a host tissue. They can be identified from other cells within a tumour by the symmetry of their cell division and changes to gene expression (Rosen and Jordan, 2009). CSCs only represent a small subpopulation of cells within a tumour, and they exhibit cell surface markers, including CD44, CD24 and CD133 (Yu et al., 2012). The evolution of the CSC is illustrated.
Under specific conditions, through epithelial mesenchymal plasticity, cells are able to sustain epithelial cell and mesenchymal stem cell characteristics to form epithelial-derived tumour cells, which then further differentiate to form CSC features (Pinto et al., 2013). These cells can have serious implications on the treatment of tumours since they exhibit resistance to chemotherapy. Chemoresistance has been demonstrated in a number of tumours, including breast and ovarian cancer (Pinto et al., 2013; Steg et al., 2012).
Conclusion
This paper has provided an overview of the genetics of cancer, as well as the cells involved and the mutations that can occur causing this the disease. Due to the complexity of the disease, a cure has not yet been found; however, it may be possible in the future as more is learned about the pathways of cancer.
Acknowledgments
I would like to express my gratitude and thanks to the government of Saudi Arabia, especially University of Tabuk, for the support. I would also like to thanks Department of Biology, Faculty of Sciences, Saudi Digital Library and University Library providing the facility for literature survey and collection.
References
- Anand P., Kunnumakara A., Sundaram C., Harikumar K., Tharakan S., Lai O., Sung B & Aggarwal B. Cancer is a preventable disease that requires major lifestyle changes. Pharmaceutical Research. 2008;25:2097-2116.
CrossRef - Deans A. J & West S. C. DNA interstrand crosslink repair and cancer. Nature Reviews Cancer. 2011;11:467-480.
CrossRef - Diekman B. O., Christoforou N., Willard V. P., Sun H., Sanchez-Adams J., Leong K. W & Guilak F. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proceedings of the National Academy of Sciences. 2012;109:19172-19177.
CrossRef - Fox I. J & Duncan S. A. Engineering liver tissue from induced pluripotent stem cells: A first step in generating new organs for transplantation? Hepatology. 2013;58:2198-2201.
CrossRef - Kesanakurti D., Maddirela D. R., Chittivelu S., Rao J. S & Chetty C. Suppression of tumour cell invasiveness and in vivo tumour growth by microRNA-874 in non-small cell lung cancer. Biochemical and Biophysical Research Communications. 2013;434:627-633.
CrossRef - Luo Y., Tsuchiya K. D., Park D., Fausel R., Kanngurn S., Welcsh P., Dzieciatkowski S., Wang J & Grady W. M. RET is a potential tumour suppressor gene in colorectal cancer. Oncogene. 2013;32:2037-2047.
CrossRef - Nguyen L. V., Vanner R., Dirks P & Eaves C. J. Cancer stem cells: An evolving concept. Nature Reviews Cancer. 2012;12:133-143.
CrossRef - National Institutes of Health. Are stem cells involved in cancer? [Online]. NIH. Available at: http://stemcells.nih.gov/info/Regenerative_Medicine/pages/2006chapter9.aspx. Accessed 29th November. 2013.
- Pedone K., Sells J & Der C. Mutational activation of KRAS and BRAF in. 2013.
CrossRef - Pinto C. A., Widodo E., Waltham M & Thompson E. W. Breast cancer stem cells and epithelial mesenchymal plasticity – Implications for chemoresistance. Cancer Letters. 2013;341:56-62.
CrossRef - Ponder B. A. J. Cancer genetics. Nature. 2001;411:336-341.
CrossRef - Popat K., Mcqueen K & Feeley T. W. The global burden of cancer. Best Practice & Research Clinical Anaesthesiology. 2013;27:399-408.
CrossRef - Rosen J. M & Jordan C. T. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670-1673.
CrossRef - Schuldt A. Meiosis ‘Reigning in’ meiotic DNA repair. Nature Reviews Molecular Cell Biology. 2013;14:132-132.
CrossRef - Siegel R., Naishadham D & Jemal A. Cancer statistics 2012. CA A Cancer Journal for Clinicians. 2012;62:10-29.
CrossRef - Stavridis M. Embryonic stem cells: A signalling perspective. In: St. John J. C. (ed.) Mitochondrial DNA Mitochondria Disease and Stem Cells. Humana Press. 2013.
CrossRef - Steg A. D., Bevis K. S., Katre A. A., Ziebarth A., Dobbin Z. C., Alvarez R. D., Zhang K., Conner M & Landen C. N. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clinical Cancer Research. 2012;18:869-881.
CrossRef - Takahashi K & Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140:2457-2461.
CrossRef - Visvader J. E. Cells of origin in cancer. Nature. 2011;469:314-322.
CrossRef - Vurusaner B., Poli G & Basaga H. Tumour suppressor genes and ROS: Complex networks of interactions. Free Radical Biology and Medicine. 2012;52:7-18.
CrossRef - Wong V. W., Sorkin M & Gurtner G. C. Enabling stem cell therapies for tissue repair: Current and future challenges. Biotechnology Advances. 2013;31:744-751.
CrossRef - Yu Z., Pestell T. G., Lisanti M. P & Pestell R. G. Cancer stem cells. The International Journal of Biochemistry & Cell Biology. 2012;44:2144-2151.
CrossRef - Zeng H., Irwin M., Lu L., Risch H., Mayne S., Mu L., Deng Q., Scarampi L., Mitidieri M., Katsaros D & Yu H. Physical activity and breast cancer survival: An epigenetic link through reduced methylation of a tumour suppressor gene L3MBTL1. Breast Cancer Research and Treatment. 2012;133:127-135.
CrossRef - Zhou J., Yue W & Pei X. Advances in cell lineage reprogramming. Science China Life Sciences. 2013;56:228-233.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





