How to Cite | Publication History | PlumX Article Matrix
Molecular Targeted Therapy for Breast Cancer: A New Frontiers
Osama Al-Amer1, Atif Abdulwahab A. Oyouni2, and Shalini Saggu2
1Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, University of Tabuk, Tabuk, Saudi Arabia.
2Department of Biology, Faculty of Sciences, University of Tabuk, Tabuk, Saudi Arabia.
Corresponding Author Email: oalamer@ut.edu.sa
DOI : http://dx.doi.org/10.13005/bbra/2529
ABSTRACT: Cancer has become a major public health problem worldwide. Researches focus on the new approaches for cancer treatments that involve the specific targets of the cancer disease. Breast cancer is the most frequent type of cancer among women, and it causes approximately 25% of the deaths in women below the age of 35. Multiple environmental and hereditary factors are responsible for breast cancer such as age, family history, postmenopausal obesity, early menarche, late menopause, alcohol consumption, pregnancy and the use of exogenous hormones. Treatment of breast cancer patients relies primarily on surgery followed by radiotherapy and systemic therapy. Several molecules expressed and secreted by breast cancer cells have been identified by their interactions, invasion and metastasis. These molecular interactions appear to maintain the cancer cells’ survival and growth. The improvement in understanding of the molecular basis of breast cancer will provide possible targets for novel therapies. Therefor, this review focuses on the molecular and cellular basis of the breast cancer treatment.
KEYWORDS: Breast cancer; prevention; radiation systemic therapy therapy;
Download this article as:| Copy the following to cite this article: Al-Amer O, Oyouni A. A. A, Saggu S. Molecular Targeted Therapy for Breast Cancer: A New Frontiers. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Al-Amer O, Oyouni A. A. A, Saggu S. Molecular Targeted Therapy for Breast Cancer: A New Frontiers. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27039 |
Introduction
Cancer is a class of diseases in which a group of cells display uncontrolled growth, invasion and sometimes metastasis to other organs. For metastasis, cancer cells can affect the processes of bone turnover by either increasing or decreasing it, which is likely due to the direct effects of the cancer cells on the processes of osteoclast Genesis or osteoblastic differentiation. Breast cancer is the most common type of cancer among women, and causes approximately 25% of deaths in women below the age of 351. Both women and men are at risk of developing breast cancer. Multiple environmental and hereditary factors are responsible for breast cancer such as age, family history, postmenopausal obesity, early menarche, late menopause, alcohol consumption, pregnancy and the use of exogenous hormones2. About 5%-10% of breast cancer cases are due to genes inherited from parents including BRCA1 and BRCA.2,3
Oestrogen and progesterone are female reproductive hormones produced naturally by the ovaries before menopause. Many physiological processes in the body such as the development and maintenance of the female sexual organs, the reproductive cycle, reproduction and various neuroendocrine functions depend on estrogen.4 These hormones affect the growth of some breast cells and increase the risk of breast cancer during the premenopausal years by interacting with their respective hormone receptors. By this binding, breast epithelial cell proliferation, central to the process of carcinogenesis (increase the cell division and DNA replication), results in random mutations, and, hence, the risk of cancer.5 These two hormones 954 AL-AMER et al., Biosci., Biotech. Res. Asia, Vol. 14(3), 953-959 (2017)
are responsible for approximately 60% of breast tumors found in premenopausal women1. Insulinlike growth factors (IGFs), epidermal growth factors (EGFs) and transforming growth factors (TGFs)-á are the most common growth factors associated with an increased risk of developing breast cancer.6
Breast cancer can also spread to other organs such as the lungs, liver, brain, and most commonly to the bones via blood vessels or the lymphatic system. The results of a 2008 UK survey indicated that approximately 20,000 women suffer from bone metastases from breast cancer. The majority of patients with advanced breast cancer develop bone metastases and suffer from longterm skeletal morbidity. Bone metastases may remain asymptomatic but pain is common and others complications include pathologic fractures, and spinal cord compression, which have a significant impact on the quality of life of patients. Increased bone turnover, imbalance and uncoupling of the processes of resorption and remodeling are the leading causes of bone metastases. Bone resorption of lytic metastases, either through direct activation of tumor cells or via tumour-secreted factors (growth factors) such as cytokines and parathyroidhormone-related peptides, are primarily caused by osteoclasts.7
Treatment of Breast Cancer
Several types of therapy can be used to treat breast cancer cells including surgery, radiation therapy and systemic therapy
Surgery
The role of surgery in breast cancer treatment involves removing cancer from the breast and lymph nodes. This type of surgery only removes the cancerous tissue plus a surrounding rim of normal tissue.
Radiation Therapy
Radiation therapy can be used to destroy the remaining cancer cells after surgery or to reduce the tumor size before surgery.
Systemic Therapy
This treatment is comprised of chemotherapy, hormone therapy and biologic therapy. Neoadjuvant therapy is a systemic therapy given to the patients before surgery to shrink the tumor as soon as possible and facilitate surgical removal. Adjuvant therapy is a systemic therapy given to the patients after surgery to kill any undetectable tumor cells that migrate to other parts of the body.
Chemotherapy is a category of cancer treatment that uses chemical substances to kill cells that divide rapidly, one of the main properties of most cancer cells. This type of therapy has some short-term side effects including myelosuppression, mucositis, hair loss and fatigue. Some long-term effect side effects of chemotherapy include cardiac dysfunction, cognitive dysfunction and leukemia.8 Single agent chemotherapy is clearly effective in causing tumor regression, but effective combination chemotherapy provides more responses and a longer duration of response. The most effective combination regimens at present contain doxorubicin. Patients with estrogen receptor positive tumors will typically receive a hormonal therapy such as anti-oestrogen therapy after chemotherapy is completed. In more advanced and progressive cases, patients will receive a targeted therapy such as anti-angiogenesis therapy or anti-bone metastasis therapy.
Successfully to treat the breast cancer targets stimulating breast cancer should be blocked. These targets can be divided into anti-oestrogen therapy (endocrine therapy),9-12 anti-growth factor therapy,13,14 anti-bone metastasis therapy15 and anti-angiogenesis therapy (see Figure 1). Anti
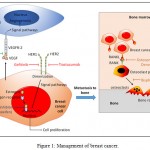 |
Figure 1: Management of breast cancer.
|
Anti-Oestrogen Therapy (Endocrine Therapy)
Endocrine therapy is one of the primary modalities of medical treatment for breast cancer. It works successfully in ‘estrogen receptor (ER) positive women’s breast cancer’.9,10,16
Table 1: Summary of breast cancer management therapy (table generated by authors)
| Target | Drug Name | Drug Mechanism |
| Oestrogen | Zoledex, buserelin | Luteinizing hormone-releasing hormone (LHRH) agonist |
| Tamoxifen | Antagonist of the estrogen receptor | |
| Raloxifene | Oestrogen receptor modulator | |
| Anastrozole, letrozole, exemestane | Aromatase inhibitors: inhibit the formation of the estrogen hormone in postmenopausal women | |
| Human epidermal growth factor receptor 1 (HER1) | Gefitinib | Tyrosine kinase inhibitor |
| Human epidermal growth factor receptor 2 (HER2) | Trastuzumab | Humanized monoclonal antibody against HER2 |
| Osteoclasts (bone resorption cells) | Bisphosphonates (clodronate, pamidronate, ibandronate, zoledronic acid) | Inhibit osteoclasogenesis and bone resorption in bone metastasis.
|
| RANKL inhibitors (osteoprotegerin, denosumab) | Block the binding of RANKL to RANK, inhibit osteoclasogenesis and bone resorption in bone metastasis | |
| Angiogenesis (new blood vessel formation) | Bevacizumab | Humanized monoclonal antibody directed against the VEGF-A ligand to inhibitor angiogenesis |
LHRH Agonist
Normally, the hypothalamus produces luteinizing hormone-releasing hormone (LHRH) to control the secretion of follicle-stimulating hormones and luteinizing hormones by the pituitary gland, and, hence, the production of gonadal steroid hormones5. Using LHRH agonists such as zoledex and buserelin results in sustained suppression of the follicle-stimulating hormone (FSH) and luteinizing hormone (LH) releases that are secreted from the pituitary gland into the ovary gland, followed by a reduction in serum estradiol, serum estrogen and serum progesterone which are secreted by the ovary glands. This type of therapy is used in the treatment of hormone-responsive metastatic breast cancer in premenopausal women.11,17
Tamoxifen
Tamoxifen is an orally selective estrogen receptor modulator (SERM), used by the National Surgical Adjuvant Breast and Bowel Project (NSABP B) Breast Cancer Prevention Trial (P-1). Generally, Estrogens bind the E region in the ER (ERá and ERâ) inducing an allosteric conformational change, which facilitates dimerization of the receptor. Then, the transcription activation of many genes involved in proliferative signaling and cell cycle progression will occur by binding to the receptor dimer with a high-affinity DNA such as cyclin D1, c-fos, c-Jun, c-myc and c-myb. In treatment, tamoxifen binds with ER as a target to stop the activation, translocation and dimerization of the ER that subsequently inhibits the transcription activation of the genes in proliferative signaling and cell cycle progression.6,12,18
Raloxifene
Raloxifene (a preventative chemotherapy for women found to have a high risk of developing breast cancer) is an oral SERM which presents an increased risk for major coronary events. Raloxifene will reduce the potential for invasive breast cancer and vertebral fractures, but the risk of cardiovascular events is not changed. Raloxifene also binds with ER as a target and reduces the incidence of ER-positive breast cancers.19
Aromatase Inhibitors
Aromatase inhibitors are drugs used in the treatment of breast cancer and work by inhibiting the formation of the estrogen hormone in postmenopausal women. Estradiol is the most potent endogenous estrogen and is commonly formed from androgens by the P-450 aromatase enzyme, which converts androgen to estradiol. Thus, aromatase inhibitors compete with the androstenedione substrate for noncovalent binding to the active site of the P-450 aromatase enzyme to inhibit the estrogen formation.4,12
On the other hand, anti-oestrogen therapy (endocrine therapy) has some adverse side effects that may be responsible for the failure of antiendocrine treatments in metastatic breast cancer. Firstly, the skeletal homeostasis in both men and women is regulated by estrogen. Also, estrogen is prescribed for the prevention of postmenopausal bone loss and reduces the incidence of osteoporotic fracture in postmenopausal women. Thus, the use of endocrine therapy will increase bone loss in postmenopausal women.20 Secondly, several recent reviews indicate the protective effect of estrogens against neurodegenerative disease in humans. Oestrogen protects against stroke, Parkinson disease and Alzheimer disease. The use of raloxifene suggests an increase in the risk of this disease.20 Thirdly, the National Surgical Adjuvant Breast and Bowel Project in North America (NSABP P-1), stated that the use of tamoxifen had been associated with the increased risk of many diseases such as uterine cancer, stroke, and venous thromboembolic events in postmenopausal women. Tamoxifen is metabolized in the liver, so its efficacy can depend on liver function.19 Fourthly, tamoxifen is responsible for a high rate of thromboembolism and a greater risk of developing endometrial cancer in women21. Fifthly, according to various studies, the interaction of ER can be altered by the phosphorylation of ER and its coregulators that may augment their transcriptional activity in a ligand-independent mode, even in the presence of SERMs like tamoxifen.22 Sixthly, SERMs such as tamoxifen can activate ‘nongenomic’ ER action and membrane-initiated steroid signalling (MISS) outside the nucleus that leads to phosphorylation, and as a result of this, activation of surface tyrosine kinase receptors such as the insulin-like growth factor I receptor (IGF-IR), the epidermal growth factor receptor (EGFR) and the HER2. Activation of these receptors leads to sustained breast cancer growth and progression.22 Finally, resistance to endocrine therapies also plays a role in the failure of anti-endocrine treatments in metastatic breast cancer that require refinements of other treatment approaches.22
Anti-Growth Factor Therapy
Receptor tyrosine kinase (RTKs) is a high-affinity cell surface receptor that plays a major role in controlling apoptosis, differentiation and proliferation processes by its signal activity. Any abnormal activation of PTKs induces a critical role in the development and progression of breast cancer. Thus, these types of receptors can serve as therapeutic targets for abnormal PTKs activation.13,14,23 primary breast cancer, it is found that 50% to 90% of TGF-á and amphiregulin ligands bind with EGFR, inducing an allosteric conformational change, which facilitates dimerization of the RTKs and their signals. Thus, gefitinib inhibits EGFR, which subsequently inhibits the signal transduction cascade of EGFR, resulting in malignant cells inhibited.23,24
Gefitinib (Iressa)
Gefitinib is a PTK inhibitor for epidermal growth factor receptor’s (EGFR) tyrosine kinase domain. EGFR is a transmembrane receptor that plays important roles in development, differentiation, proliferation and migration. In primary breast cancer, it is found that 50% to 90% of TGF-á and amphiregulin ligands bind with EGFR, inducing an allosteric conformational change, which facilitates dimerization of the RTKs and their signals. Thus, gefitinib inhibits EGFR, which subsequently inhibits the signal transduction cascade of EGFR, resulting in malignant cells inhibited.23,24
Herceptin
Trastuzumab (commonly known as herceptin) is a humanized monoclonal antibody against HER2 in HER2-positive metastatic breast cancer. HER2/neu stands for ‘human epidermal growth factor receptor 2’ and plays important roles in the development, differentiation, proliferation and migration of breast cancer cells. Thus, HER2 is a target for herceptin, which works on both the extracellular and intracellular domains of the receptor.25,26
Anti-Bone Metastasis Therapy
Bone metastasis from breast cancer is the most common form of morbidity, and affects 65% to 75% of women with advanced breast cancer. Bone lesions can induce skeletal complications or skeletal-related events (SREs) such as pathologic fractures, spinal cord compression, radiation or surgery to the bone and potentially life-threatening hypercalcemia of malignancy (HCM) that spread to more than one site, often need many years of treatment and are incurable27. To manage bone metastasis from breast cancer, it is suggested that effective therapies such as bisphosphonates and the receptor activator of nuclear factor-êB ligand (RANKL) inhibitors which inhibit bone resorption can reduce the risk of skeletal complications.15,28
Bisphosphonates
Currently, bisphosphonates are a standard therapy used to prevent skeletal complications associated with bone metastases. They bind to the bone and are released during bone resorption from the bone matrix to inhibit osteoclast activity and survival. In particular, bisphosphonates absorbed by osteoclasts induce apoptosis in osteoclasts and inhibit osteoclastogenesis. Clodronate, pamidronate, ibandronate and zoledronic acid are bisphosphonates approved for the treatment of patients with bone metastases from breast cancer.28,29
Zoledronic acid is a newer nitrogencontaining bisphosphonate which has a unique mechanism and increased clinical activity compared to the first-generation bisphosphonates such as etidronate and clodronate. By comparing zoledronic acid directly with pamidronate, zoledronic acid showed significantly more efficient results in reducing the risk of SREs, which was 20% more than pamidronate among breast cancer patients. Also, a 4 mg zoledronic acid via 15 min infusion every four weeks for one year has been compared with a placebo in 227 Japanese women with breast cancer-related bone metastases. This study indicated a significant (39%) reduction in the rate of SREs for patients who received zoledronic acid in their treatment when compared with patients who received a placebo in their treatment.28
RANKL Inhibitors
The receptor activator of nuclear factor-êB (RANK) is a membrane protein that is expressed on the surface of osteoclasts which is involved in the activation of osteoclasts. The binding between RANK and RANKL mediates bone resorption and the release of growth factors from bone matrix, resulting in a cycle of bone breakdown and tumor proliferation. Thus, RANKL plays an essential role in the formation, function and survival of osteoclasts.30,31
Osteoprotegerin (Fc-OPG) is an antiRANKL therapy which inhibits bone resorption in bone metastasis. It works by neutralizing the RANKLs’ biological effects and blocking the association of RANKL or any other ligands with RANK. In 2008, Jeroen et al. reported that Fc-OPG was shown to be a very potent agent in reducing intra-bone tumor burden, but it failed to reduce total tumor burden which included extramedullary growth.32
Denosumab is a human monoclonal antibody which blocks the binding of RANKL to RANK in postmenopausal metastatic breast cancer patients. In 2004, Bekker et al. reported that denosumab is a particular anti-RANKL agent which induces a dose-dependent rapid, sustained decrease from baseline in bone turnover, and that it could be used as an effective, convenient treatment for osteoporosis. Additionally, denosumab is considered safer and more efficacious than FcOPG as a therapeutic inhibitor of RANKL because binding of Fc-OPG to TNF-related apoptosisinducing ligands could inhibit its role in tumor surveillance.31,33
Angiogenesis in Breast Cancer
Angiogenesis is a physiological process of new blood vessel formation that plays a central role in both local tumor growth and distant metastasis in breast cancer. During active angiogenesis, the matrix metalloproteinase (MMP) will be increased, degrading the basement membrane and extracellular matrix. Some angiogenic proteins and growth factors responsible for the stimulation of angiogenesis are released from breast cancer cells such as the vascular endothelial growth factor (VEGF) which plays a crucial role in the formation of blood vessels that lead to tumor growth and allows it to expand.34 To treat or manage the angiogenesis in breast cancer various drugs can be used. Currently, bevacizumab is the most therapeutic agent specifically designed to disrupt angiogenesis. It is a humanized monoclonal antibody directed against the VEGF-A ligand34.
References
- Sharma R., Beith J., Hamilton A. Systematic review of LHRH agonists for the adjuvant treatment of early breast cancer. Breast. 2005;14(3):181-91.
CrossRef - Althuis M. D., et al. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1558-68.
- Yang X., Lippman M. E. BRCA1 and BRCA2 in breast cancer. Breast Cancer Res Treat. 1999;54(1):1-10.
CrossRef - Brueggemeier R. W., Hackett J. C., Diaz-Cruz E. S. Aromatase inhibitors in the treatment of breast cancer. Endocr Rev. 2005;26(3):331-45.
CrossRef - Spicer D. V., Pike M. C. Future possibilities in the prevention of breast cancer: luteinizing hormone-releasing hormone agonists. Breast Cancer Res. 2000;2(4):264-7.
CrossRef - Hamelers I. H., Steenbergh P. H. Interactions between estrogen and insulin-like growth factor signaling pathways in human breast tumor cells. Endocr Relat Cancer. 2003;10(2):331-45.
CrossRef - Fallowfield L., Jenkins V., Coleman R. Which bisphosphonates do oncologists prescribe for women with metastatic breast cancer and why? Results of a UK survey. Breast. 2008;17(5):459-63.
CrossRef - Partridge A. H., Burstein H. J., Winer E. P. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. J Natl Cancer Inst Monogr. 2001;30:135-42.
CrossRef - Andreucci E., et al. Targeting the receptor tyrosine kinase RET in combination with aromatase inhibitors in ER positive breast cancer xenografts. Oncotarget. 2016;7(49):80543-80553.
CrossRef - Bacci M., et al. miR-155 Drives Metabolic Reprogramming of ER+ Breast Cancer Cells Following Long-Term Estrogen Deprivation and Predicts Clinical Response to Aromatase Inhibitors. Cancer Res. 2016;76(6):1615-26.
CrossRef - Mastro D . L., et al. New insights on the role of luteinizing hormone releasing hormone agonists in premenopausal early breast cancer patients. Cancer Treat Rev. 2016;42:18-23.
CrossRef - Lin Y. L., et al. Inhibition of breast cancer with transdermal tamoxifen-encapsulated lipoplex. J Nanobiotechnology. 2016;14:11.
CrossRef - Toy K. A., et al. Tyrosine kinase discoidin domain receptors DDR1 and DDR2 are coordinately deregulated in triple-negative breast cancer. Breast Cancer Res Treat. 2015;150(1):9-18.
CrossRef - Spanheimer P. M., et al. Receptor Tyrosine Kinase Expression Predicts Response to Sunitinib in Breast Cancer. Ann Surg Oncol. 2015;22(13):4287-94.
CrossRef - Krzeszinski J. Y., Wan Y. New therapeutic targets for cancer bone metastasis. Trends Pharmacol Sci. 2015;36(6):360-73.
CrossRef - Pritchard K. Endocrinology and hormone therapy in breast cancer: endocrine therapy in premenopausal women. Breast Cancer Res. 2005;7(2):70-6.
CrossRef - Lambertini M., et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol. 2015;26(12):2408-19.
CrossRef - Cuzick J., et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67-75.
CrossRef - Grady D., et al. Reduced incidence of invasive breast cancer with raloxifene among women at increased coronary risk. J Natl Cancer Inst. 2008;100(12):854-61.
CrossRef - Deroo B. J., Korach K. S. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561-70.
CrossRef - Gradishar W. J. Tamoxifen–what next? Oncologist. 2004;9(4):378-84.
CrossRef - Massarweh S., Schiff R. Unraveling the mechanisms of endocrine resistance in breast cancer new therapeutic opportunities. Clin Cancer Res. 2007;13(7):1950-4.
CrossRef - Camirand A., et al. Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast Cancer Res. 2005;7(4):570-9.
CrossRef - Bellizzi A., et al. The scaffolding protein NHERF1 sensitizes EGFR-dependent tumor growth, motility and invadopodia function to gefitinib treatment in breast cancer cells. Int J Oncol. 2015;46(3):1214-24.
CrossRef - Piccart-Gebhart M. J., et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659-72.
CrossRef - Noh J. K., et al. Herceptin-functionalized pure paclitaxel nanocrystals for enhanced delivery to HER2-postive breast cancer cells. Int J Pharm. 2016;513(1-2):543-553.
CrossRef - Clemons M. J., et al. Phase II trial evaluating the palliative benefit of second-line zoledronic acid in breast cancer patients with either a skeletal-related event or progressive bone metastases despite first-line bisphosphonate therapy. J Clin Oncol. 2006;24(30):4895-900.
CrossRef - Coleman R. E. Bisphosphonates in breast cancer. Ann Oncol. 2005;16(5):687-95.
CrossRef - Hadji P. et al. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Ann Oncol. 2016;27(3):379-390.
CrossRef - Criscitiello C., et al. Crosstalk between bone niche and immune system: osteoimmunology signaling as a potential target for cancer treatment. Cancer Treat Rev. 2015;41(2):61-68.
CrossRef - Ottewell P. D., et al. OPG-Fc inhibits ovariectomy-induced growth of disseminated breast cancer cells in bone. Int J Cancer. 2015;137(4):968-977.
CrossRef - Buijs J. T., et al. Inhibition of bone resorption and growth of breast cancer in the bone microenvironment. Bone. 2009;44(2):380-386.
CrossRef - Bekker P. J., et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19(7):1059-66.
CrossRef - Schneider B. P., Miller K. D. Angiogenesis of breast cancer. J Clin Oncol. 2005;23(8):1782-1790.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





