How to Cite | Publication History | PlumX Article Matrix
Pramod Kumar Mahish1 and Anjali Ghritlahare2
1Department of Biotechnology, Govt. Digvijay Post Graduate (Autonomous) College Rajnandgaon (Chhattisgarh), India.
2Krishi Vigyan Kendra (KVK) Kanker, Affiliated with Indira Gandhi Agriculture University Raipur (Chhattisgarh), India.
Corresponding Author E-mail: drpramodkumarmahish@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2561
ABSTRACT: The Chrysanthemum also known as mums or chrysenths, are useful in ornamental applications, insecticidal, air pollution reducer, perfume production etc. The Phoma causes infection to Chrysanthemum which results in serious lesion. The present study reports protection of Chrysanthemum against fungal infection. The morphology of Phoma chrysanthemicola was studied in PDA, CZA and MEA medium. The pathogenicity of fungus was examined on different variety of chrysanthemum plants. The control of P. chrysanthemicola was contemplated by utilizing some commercial available fungicides and extract of medicinal plants. The organism causes root rot and ray blight to Chrysanthemum plants. Chrysanthemum sp. 2 was found more sensitive to pathogen took after by Chrysanthemum sp. 1 and 3. Relationship between diseases severity and incubation period of pathogen with various chrysanthemum plant was found R2 0.95, 0.97 and 0.87 for Chrysanthemum sp. 1, 2 and 3. Carbendazim was recorded more effective on P. chrysanthemicola followed by mancozeb and zineb with P value of 0.065 at 0.05 level. Azadirachta indica extract and plant extract from methanolic solvent were found more effective against P. chrysanthemicola. Now it is presumed that fungal pathogen has strong ability to infect chrysanthemum but chemical and biological alternate can control the chrysanthemum against pathogen.
KEYWORDS: Azadirachta indica; Chrysanthemum; Carbendazim;Phoma Chrysanthemicola;Ocimum tenuiflorum
Download this article as:| Copy the following to cite this article: Mahish P. K, Ghritlahare A. Pathogenicity of Phoma Chrysanthemicola to Chrysanthemum Plants (Asteraceae Family) and Control of Pathogen by Chemical and Biological Approach. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Mahish P. K, Ghritlahare A. Pathogenicity of Phoma Chrysanthemicola to Chrysanthemum Plants (Asteraceae Family) and Control of Pathogen by Chemical and Biological Approach. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27768 |
Introduction
The genus Chrysanthemums have a place with the family Asteraceae originated mainly from East Asia. It is cultural symbol in China, Japan and Korea. Chrysanthemums are cultivated commercially in Jammu and Kashmir, Assam, Karnataka, Kerala and found throughout the country. The different part of Chrysanthemum contain phytochemical constituents, which includes pyrenthroids, sesquiterpenoids, flavonoids, coumarins, triterpenoids, steroids, phenolics, purines, lipids aliphatic compounds and monoterpenoids. The phytochemical constituents of Chrysanthemum are biological active such as molluscicidal, cytotoxicity, insecticidal etc. The bioactive compounds are likewise useful in pharmaceutical purposes.1 Chrysanthemum are active against Herpes simplex virus, antibacterial, antioxidant and anti-aging.2,3,4 The Chrysanthemum extract additionally discovered defensive against the tumour. It represses the development of hepatocellular carcinoma and prostate cancer.5,6,7
The genus Phoma belongs to Coelomycetes. It is common soil fungi but a wide group of Phoma infects the different plants. The infection of P. chrysanthemicola to chrysanthemum plant was observed few decades ago.8,9,10 But in recent ray blight of Pyrenthrum (now classified in Chrysanthemum) was observes, which was infected by Phoma.11,12 Phoma chrysanthemicola and some other new species of Phoma genus recently investigated from important crop worldwide which now making an attraction towards the management of P. chrysanthemicola.13,14,15
Some fungicides were previously used for the management of ray blight of plants infected by the Phoma. The fungicides give noteworthy decrease in mycelial development in vitro16. Extract of some medicinal plants was additionally utilized as broad – spectrum antifungal. The extract of tulsi (Ocimum tenuiflorum) was found to control the growth of Candida albicans,17 Fusarium solani,18 Aspergillus niger19 and A. flavus.20 Similarly, extract of neem (Azadirachta indica) was also used to control the infection caused by Aspergillus, Rhizopus, A. fumigatus, Candida albicans, Fusarium oxysporum, Phoma tarda, Rhizoctonia solani and Hemileia vastatrix.21,22,23
Materials and Methods
The Chrysanthemums are very important and valuable plant given by nature to us. So it is extremely important to secure it against fungal pathogen like Phoma. The present work, therefore, aims to understand the characteristics of fungal pathogen, its infection and disease causing ability and the control of pathogen using chemical and biological approach. Following methodologies were applied to achieve the above goal.
Morphological Study of Fungus
The organism Phoma chrysanthemicola was obtained from School of studies in Biotechnology, Pt. Ravishankar Shukla University Raipur, India which was isolated from our previous study27. The organism was maintained in Potato dextrose agar slant for further study. Czapek dox agar (CZA), Potato dextrose agar (PDA) and Malt extract agar (MEA) medium were chosen to grow fungus for study of morphological characteristics. The plates were maintained at 26°C for one week. Morphological characteristics such as form, margin, surface, color, pigment and diameter of fungi were observed every day up to day 7. After this Lactophenol cotton blue stain was used to observe the fungi in microscope. The microphotograph of fungus was taken by Labomad image device (digi pro software 2.0) inbuilt in binocular compound microscope.
Disease Causing Ability of P. Chrysanthemicola
Three different Chrysanthemums were taken in the present work named as Chrysanthemum sp. 1, 2 and 3.Plants were artificially infected with a suspension of pycnidiospore when the plant achieved 5-6 week old. Pycnidiospore suspension was set up as already portrayed by Onfroy et al.,24 with little modification. Ten day old culture was used to prepare inoculums. Sterile distilled water flooded to fungal culture and then colony was scraped by glass rod took after by filtration of suspension. Then Tween 20 was added as wetting agent. Pycnidiospore suspension was then applied to plant with hand sprayer. The inoculums were applied for three times in interval of 12hr.
In the wake of spraying of inoculums severity and intensity of fungal disease was studied. Disease severity was assessed using 0 to 5 point scale method. The technique was already depicted by Tivoli et al.,25 The 0 to 5 point scale includes, 0= no lesion; 1= a few scattered flecks; 2= numerous flecks; 3= 10–15% of leaf area necrotic and appearance of coalesced necrosis; 4= 50% of the leaf area dehydrated or covered by lesions; 5= 75–100% of the leaf area dehydrated or necrotic. Three leaves were arbitrarily chosen from plant and considered by to 5 point scale in interim of 5 days started from fifth day and ended to the 40 days. Percent of disease intensity (PDI) was calculated using the formula –
Effect of Fungicides on Growth of P. Chrysanthemicola
Effect of some commonly available fungicides was tested against P. chrysanthemicola. Carbendazim (50% WP), Mencozeb (63% WP) and Zineb (75% WP) were used in the present study. Set of flasks with 50 ml Potato dextrose broth (PDB) medium containing fungicide concentration of 0.2, 0.4 and 0.6 mg/l were autoclaved. After preparation of flasks, P. chrysanthemicola was inoculated to the medium (1 ml, 72 hr old inoculums cultured in PDB medium). Flasks were then incubated to 26±1°C for 7 days. All concentration was taken into triplicate. After incubation period, mycelium were filtered by muslin cloth and dried in oven at 80°C for 10 hours. Percent of growth inhibition was calculated by comparing the biomass with control. The methodology was modified from some previous works.26,27
Effect of Plant Extract on Growth of P. Chrysanthemicola
The mature plant leave sample of Neem (Azadirachta indica) and Tulsi (Ocimum tenuiflorum) were collected from Botanical Garden of Govt. Digvijay PG College Rajnandgaon (C.G.). The extraction of leaf samples was done according to Mondallet al.,21 As per the protocol plant leave samples were surface sterilized with tap water followed by sterile distilled water and air dried at room temperature. Pre-weighted leaf samples (20g) were then ground in the sterile mortar with 20 ml Acetone and Methanol respectively. The extracted samples were then allow to stand for 1hr. Samples were then filter through fine cloth for three times. Three flasks were prepared with PDB medium containing 5% and 10% vol/vol extracted samples of Neem (Azadirachta indica) and Tulsi (Ocimum tenuiflorum) individually. One flask was left as control which was without leaf extract. Fungus inoculums were inoculated to the medium as described previously. All concentrations were taken in triplicates which were then incubated to 26±1°C for 7 days. After incubation period mycelium were filtered, dried and impact of plant concentrate on development of organism was recorded as portrayed in the approach of impact of fungicides on development of P. chrysanthemicola given already.
Results
Morphological and Microscopic Characteristics of P. Chrysanthemicola
Phoma chrysanthemicolais moderate growing fungi however contrasts in morphological properties were observed in different growth medium. It was filamentous in CZA while circular in PDA and MEA. Difference in colour, margin and surface was likewise seen in various medium. Most extreme development was recorded in PDA (73mm) and least growth was seen in CZA medium (56mm). Its growth was found flat on the medium, the surface was observed as smooth to cottony. The front color was found dark green to brown and the back shading was observed as dark brown to black (Figure 1). Phoma produces their conidia (spore) in encased structure called pycnidia. The pycnidia were found pyriform to globus (100 to 400µm) in brown to black color. The conidia were found ovoid to ellipsoidal (5-10 µm). The mature conidia discharged from interior to pore opening site (Figure 2).
 |
Figure 1: Growth of P. chrysenthemicola on different growth medium (A) Front view of fungus in CZA (B) Back view of fungus in CZA (C) Front view of fungus in MEA (D) Back view of fungus in MEA (E) Front view of fungus in PDA (F) Back view of fungus in PDA
|
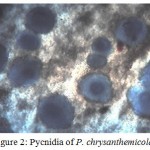 |
Figure 2: Pycnidia of P. chrysanthemicola
|
Infection of P. Chrysanthemicola to Chrysanthemum Plant
Three Chrysanthemum plants were infected by P. chrysanthemicola suspension spray. Distinctive disease intensity was seen amid examination. The disease intensity was increased simultaneously with incubation period. The mean of point scaling was found 1.6 at 20th day of incubation took after by 2.6 (25th day), 3.6 (30th day), 4.6 (35th day) and 4.8 (40th day) on Chrysanthemum sp 1. Likewise the mean of point scaling of Chrysanthemum sp 2 was found 5.0 at 40th day of incubation and 3.8 was recorded for Chrysanthemum sp 3. The infected plants are showing in the Figure 3, 4,5,6,7 and 8 and the regression analysis between disease severity of plant and incubation of fungal pathogen is presented in Figure 9. The value of R2 (Coefficient of determination) for the relationship between disease severity and incubation period (R2 = 0.958) indicates that 95.8% of the disease severity of Chrysanthemum sp. 1 is explained by incubation period of fungal pathogen. Similarly the value of R2 (Coefficient of determination) for Chrysanthemum sp. 2 and 3 was obtained 97.1 and 87.3%.
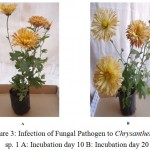 |
Figure 3: Infection of Fungal Pathogen to Chrysanthemum sp. 1 A: Incubation day 10 B: Incubation day 20
|
 |
Figure 4: Infection of Fungal Pathogen to Chrysanthemum sp. 1 A: Incubation day 30 B: Incubation day 40
|
 |
Figure 5: Infection of Fungal Pathogen to Chrysanthemum sp. 2 A: Incubation day 10 B: Incubation day 20
|
 |
Figure 6: Infection of Fungal Pathogen to Chrysanthemum sp. 2 A: Incubation day 30
|
 |
Figure 7: Infection of Fungal Pathogen to Chrysanthemum sp. 3 A: Incubation day 10 B: Incubation day 20
|
 |
Figure 8: Infection of Fungal Pathogen to Chrysanthemum sp. 3 A: Incubation day 10 B: Incubation day 20
|
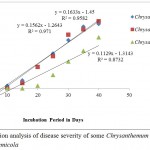 |
Figure 9: Regression analysis of disease severity of some Chrysanthemum plants infected by Phoma chrysenthemicola
|
The disease intensity of Chrysanthemum sp. 1 was found 60.00 at 15th day of incubation followed by 80.00 and 86.67 (20th and 25th day), 90.00 (30th day), 92.00 (35th day) and 96.00 at 40th day of incubation. Similarly the disease intensity of Chrysanthemum sp 2 was found 100.00 at 40th day of incubation while 76.00 PDI was found for Chrysanthemum sp 3 (Figure 10). Percent of disease intensity (PDI) caused by P. chrysanthemicola to Chrysanthemum sp. 1, 2 and 3 were subjected to study the test of significance using one way ANOVA test at significance level of P 0.05. The p value was found 0.278 which is lower than the table value thus concluded that PDI of three different plants have different effect. There is significant different among PDI of Chrysanthemum sp. 1, 2 and 3 caused by P. chrysanthemicola. In the ANOVA error result Chrysanthemum sp. 1 has significant higher mean (65.58) followed by chrysanthemum sp. 2 (56.63) and 3 (35.33).
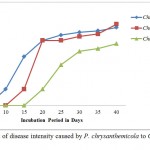 |
Figure 10: Percent of disease intensity caused by P. chrysanthemicola to Chrysanthemum sp. 1, 2 and 3
|
The disease caused by P. chrysanthemicola to three species of Chrysanthemum is presented in the table 1. The diseases of Chrysanthemum plants were identified by Plant Pathology department, Krishi Vigyan Kendra, Kanker (Chhattisgarh) India affiliated to Indira Gandhi Agriculture University Raipur (Chhattisgarh) India.
Table 1: Disease caused by Phoma chrysanthemicola to the Chrysanthemum Plants
| S. No. | Plant | Infected Part | Characteristics/ symptoms | Disease |
| 1 | Chrysanthemum Sp. 1 | Leaf | Small spots in early stage on lower leaves leading to large dark brown spot at later | Ray blight |
| Flower | Dark coloring in lower petals leading to premature dryness of flower and rot | Ray blight | ||
| Stem | No infection observed | |||
| Root | No infection observed | |||
| 2 | Chrysanthemum Sp. 2 | Leaf | Small spots in early stage on lower leaves leading to large dark brown spot at later | Ray Blight |
| Flower | Dark coloring in lower petals leading to premature dryness of flower and rot | Ray Blight | ||
| Stem | Green in early stage leading to browning and wilt | |||
| Root | Dark brown necrotic region on the root, wilt and immature death of plant, root rot and some part of root remain in soil wile separating the root from pot | Root rot | ||
| 3 | Chrysanthemum Sp. 3 | Leaf | Dark spot on leaf observed at later stage only | |
| Flower | No infection observed | |||
| Stem | No infection observed | |||
| Root | No infection observed |
Effect of Fungicides on Growth of P. Chrysanthemicola
The effect of fungicides on growth of P. chrysanthemicola was studied. Carbendazim, mencozeb and zineb were added to the medium and after incubation dry weight of mycelium were measured. Only 0.2mg/l concentration of carbendazim inhibited the 89.04 percent growth of P. chrysanthemicola. As the concentration increases to 0.4 to 0.6mg/l the development was totally restrained and the percent of inhibition was recorded 96.36 and 99.36. So also 45.69 percent inhibition of growth was recorded in 0.2 mg/l concentration of mencozeb.
Percent of inhibition was recorded 65.26 mg/l and 82.66 at the concentration of 0.4 and 0.6 mg/l. The zineb inhibited 29.88, 58.06 and 75.40 percent development in 0.2, 0.4 and 0.6 mg/l concentration. So the carbendazim was found more effective against growth of P.chrysanthemicolatook after by mencozeb and zineb (Table 2).
Table 2: Effect of some fungicides on Growth of Phoma chrysanthemicola
| S. No. | Concentration
Mg/l |
Carbendazim | Mencozeb | Zineb | |||
| Weight of Mycelium
Mean ± SE of Triplicate |
Percent of Growth Inhibition | Weight of Mycelium
Mean ± SE of triplicate |
Percent of Growth Inhibition | Weight of Mycelium
Mean ± SE of triplicate |
Percent of Growth Inhibition | ||
| 1 | 0.2 | 0.187±0.049 | 89.04 | 0.927±0.025 | 45.69 | 1.197±0.083 | 29.88 |
| 2 | 0.4 | 0.063±0.028 | 96.31 | 0.593±0.025 | 65.26 | 0.716±0.036 | 58.06 |
| 3 | 0.6 | 0.011±0.011 | 99.36 | 0.296±0.016 | 82.66 | 0.420±0.019 | 75.40 |
Control 1.707±0.060, P= 0.065
Effect of fungicides Carbendazim, mencozeb and zineb on growth of P. chrysanthemicola was subjected to study the test of significance using one way anova at significance level P 0.05. In which mean of biomasses were taken as data source. The P value (p-value) was found 0.065 which is lower than the table value at significance level of 0.05 concluded use of different fungicides against growth of P. chysanthemicola have different effect. There is critical diverse among fungicides affecting the development of fungus. Study with ANOVA error demonstrate zineb has significant higher mean (1.01) compared with mencozeb (0.81) and carbendazim (0.49).
Effect of Plant Extract on Growth of P. chrysanthemicola
Neem (Azadirachta indica) and Tulsi (Ocimum tenuiflorum) extract were examined for impact on the development of P. chrysanthemicola. The acetone and methanolic extract of both plants were utilized against fungus. Acetone extract of Neem (Azadirachta indica) was inhibited 20.31 percent growth of P. chrysanthemicola in 5% vol/vol concentration followed by 45.99 percent inhibition in 10% vol/vol. The methanolic extract of Neem (Azadirachta indica) inhibited 49.94 percent growth in 5% vol/vol concentration while its 10% vol/vol concentration inhibited 55.41 percent growth of P. chrysanthemicola. Similarly acetone extract of Tulsi (Ocimum tenuiflorum) restrained the fungal growth at 11.00 and 22.46 percent in 5 and 10 percent vol/vol concentration while methanolic extract inhibited 48.08 and 51.92 percent growth in same concentration (Figure 11).
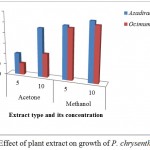 |
Figure 11: Effect of plant extract on growth of P. chrysenthemicola
|
Discussion
Hollos, in 1907 discovered Phoma chrysanthemicola, it is a common saprophytic fungi.28 Phoma is important group of fungi causing various plant diseases. Phoma causes infection to Chrysanthemum which results in blight and ray blight lesion. The ray blight of Phoma infects flower, leaf and stems of plant.11,13 Root rot is another serious infection of Phoma. It damages the root of chrysanthemum and some other plants.8,9 Comparable contamination was additionally found in present work. So, the Phoma is greater threat to the plants. The P. chrysanthemicola and some other species of Phoma were already recorded in the different habitat of Raipur and surroundings.29,30,31
So it is necessary to the control its infection. Numerous methodologies are accessible to control the infection of Phoma. Among them chemical treatment are effective yet it has some downside like its persistence and negative impact on soil fertility. Biological approaches is still not in practice so some chemical treatment like carbendazim, mencozeb and zineb were utilized to control the infection of Phoma for protection of different crops worldwide.32,33 Carbendazim and Mencozeb was likewise used to control the pathogenic fungi other than Phoma which includes Alternaria sp., Aspergillus flavus, A. niger, A. terrus, A. oryzae, A. fumigatus, Fusarium moniliforme and F. solani.34,35 The above chemical based fungicides were also used in the present work and found significant control of pathogen.
As the chemical treatment of fungi is not an ecofriendly approach so that, the scientific communities are now focusing to alternate approach towards control of pathogenic fungi through some medicinal plant extracts. Among the different medicinal plants some common and very easily available medicinal plants were utilized as a part of present work. In recent, Phoma and some other plant pathogen like Fusarium, Aspergillus were treated with extract of Neem (Azadirachta indica) and found significant outcome to control the host plant.22,23 Similarly, extract of tulsi (Ocimum tenuiflorum) was additionally used to control the Fusarium18 and Aspergillus.19,20
Conclusion
During investigation, P. chrysanthemicola was found infected to chrysanthemum plant. It causes ray blight in flower and leaves and root rot. Plant species chrysanthemum 1 and 2 were found sensitive to fungal attack while Chrysanthemum 3 was found resistant at many levels. The carbendazim was found effective against fungal growth followed by mencozeb and zineb. During the study of ecofriendly approach towards control of fungus, Azadirachta indica was discovered better alternative as contrast with Ocimum tenuiflorum extract. The methanolic solvent extract of both plants were found better inhibition of fungus as compare with extract of acetone. Now it is concluded that, P. chrysanthemicola is able to infect the chrysanthemum plant yet it is conceivable to control it from antifungal agents. Extract of common medicinal plants is likewise a contrasting option to control the growth which is an ecofriendly approach.
Conflict of Interest
Conflict of interest declared none.
Acknowledgment
The authors are thankful to Autonomous cell of Govt. Digvijay P.G. College Rajnandgaon, (C.G.) India for proving financial support for this work.
References
- Kumar A., Singh S. P., Bhakuni R. S. Secondary metabolites of Chrysanthemum genus and their biological activities. Current Science. 2005;89(9):1489-1501.
- Sassi A. B., Skhiri F. H., Bourgougnon N. A. M. Antimicrobial activities of four Tunisian Chrysanthemum species. Indian J. Med. Res. 2008;127:183-192.
- Nateepat P., Patchanee Y. Antibacterial Activity of Chrysanthemum indicum, Centella asiatica and Andrographis paniculata against Bacillus cereus and Listeria monocytogenes under Osmotic Stress. Au J.T. 2012;15(4):239-245.
- Jing S., Xiaoming Z., Liang J. Y. Antioxidant Activity, Antitumor Effect, and Antiaging Property of Proanthocyanidins Extracted from Kunlun Chrysanthemum Flowers. Oxidative Medicine and Cellular Longevity. 2015. http://dx.doi.org/10.1155/2015/983484.
CrossRef - Sang C. J., Sang M. K., Yong T. J., Chi H. S. Hepatoprotective effect of water extract from Chrysanthemum indicum L. flower. Chinese Medicine. 2013;8:1-7. doi:10.1186/1749-8546-8-7.
CrossRef - Wang Z. D., Huang C., Li Z. F., Yang J., Li B. F., Liang R.R., Dai Z. J., Lium Z. W. Chrysanthemum indicum ethanolic extract inhibits invasion of hepatocellular carcinoma via regulation of MMP/TIMP balance as therapeutic target. Oncology Reports. 2010;23(2):413-421.
- Kim C., Kim M. C., Kim S. M., Nam D., Choi S. H., Kim S. H., Ahn K. S., Lee E. H., Jung S. H., Ahn K. S. Chrysanthemum indicum L. Extract Induces Apoptosis through Suppression of Constitutive STAT3 Activation in Human Prostate Cancer DU145 Cells. Phytother. Res. 2013;27:30–38. doi: 10.1002/ptr.4689.
CrossRef - Peerally M. A. Colhoun J. The epidemiology of root rot of Chrysanthemums caused by Phoma sp. Transactions of the British Mycological Society. 1969;52(1):115–123.
CrossRef - Schneider R., Plate H. P. A root and collar rot of Chrysanthemum indicum new to Germany, caused by Phoma chrysanthemicola. Phytopathologische Zeitschrift. 1970;67:97-111.
CrossRef - Kenneth F. B., Lily H. D., Wilhelm S., William C. An aggressive vascularinhabiting Phoma (Phoma tracheiphila f. sp. chrysanthemi nov. f. sp.) weakly pathogenic to chrysanthemum. Canadian Journal of Botany. 1985;63(10): 1730-1735.
CrossRef - Pethybridge S. J., David H. G., Frank S. H. Epidemics of Ray Blight on Pyrethrum are Linked to Seed Contamination and Overwintering Inoculum of Phoma ligulicola var. inoxydabilis. Phytopathology. 2011;101(9): 1112-1121.
CrossRef - Hay F. S., Gent H. D., Pilkington S. J., Pearce T. L., Scott J. B., Prthybridge S. J. Changes in distribution and frequency of fungi associated with a foliar disease complex of Pyrethrum in Australia. Plant Disease. 2015;99: 1227-1235.
CrossRef - Davidson J. A., Hartley D., Priest M., Krysinska-Kaczmarek M., Herdina A., McKay E. S. A new species of Phoma causes ascochyta blight symptoms on field peas (Pisum sativum) in South Australia. Mycologia. 2009;101(1):120–128. DOI: 10.3852/07-199.
CrossRef - Dario I., Antem I., Tihomir M., Bogdan C. Shoot necrosis of olive caused by Phoma incompta, a new disease of olive in Croatia. Phytopathol. Mediterr. 2010;49:414−416.
- Soni R., Diwan R. Isolation of Folliicolous Necrotic Fungi from Medicinally Significant Plant Trigonella Foenum–Graecum.Indian J.Sci.Res. 2014;4(1):205-206.
- Pethybridge S. J., Hay F. S., Wilson C. R. Groom T. Development of a Fungicide Based Management Strategy for Foliar Disease Caused by Phoma ligulicola in Tasmanian Pyrethrum Fields. Plant Disease. 2005;89(10):1114-1120. http://dx.doi.org/10.1094/PD891114.
- Sharma A. MVSc. Thesis. Department of Veterinary Microbiology and Immunology, DUVASU, Mathura. UP, India. 2010.
- Joseph B., Dar M. A., Kumar V. Bioefficacy of Plant Extracts to Control Fusarium solani F. Sp. Melongenae Incitant of Brinjal Wilt. Global J. Biotechnol. Biochem. 2008;3(2):56-59.
- Tewari B. B., Robert S. Studies on the Interaction of Natural Antifungals with Metal Ferrocyanides and Their Medicinal Applications. Nature Sci. 2009;7(3):1-7.
- Reddy K. R. N., Reddy C. S., Muralidharan K. Potential of botanicals and biocontrol agents on growth and aflatoxin production by Aspergillus flavus infecting rice grains. Food Control. 2009;20:173–178.
CrossRef - Mondall N. K., Mojumdar A., Chatterje S. K., Banerjee A., Datta J. K. Gupta S. Antifungal activities and chemical characterization of Neem leaf extracts on the growth of some selected fungal species in vitro culture medium. J. Appl. Sci. Environ. Manage. 2009;13(1):49–53.
- Reddy Y., Krishna K. C., Lokanatha O., Mamatha S., Reddy C. Antimicrobial activity of Azadirachta indica (neem) leaf, bark and seed extracts. Int. J. Res. Phytochem. Pharmacol. 2013;3(1):1-4.
- Silva J. L., Souza P. E., Monteiro F. P., Freitas M. L. O., Silva M. B., Belan L. L. Antifungal activity using medicinal plant extracts against pathogens of coffee tree. Rev. Bras. Pl. Med. Campinas. 2014;16(3):539-544.
- Onfroy C., Tivoli B., Corbiere R., Bouznad Z. Cultural, molecular and pathogenic variability of Mycosphaerella pinodes and Phoma medicaginis var. pinodella isolates from dried pea (Pisum sativum) in France. Plant Pathol. 1999;48:218-229.
CrossRef - Tivoli B., Beasse C., Lemarchand E., Masson E. Effect of ascochyta blight (Mycosphaerella pinodes) on yield components of single pea (Pisum sativum) plants under field conditions. Annals of Applied Biology. 1996;129:207–216. doi: 10.1111/j.17447348.1996. tb05745.x.
- Atuanya E. I., Oseghe E. O. Lead contamination and microbial lead tolerance in soils at major road junctions in Benin City. J. Applied Sci. Environ. Manage. 2006;10:99-104.
- Mahish P. K., Tiwari K. L., Jadhav S. K. Biodiversity of Fungi from Lead Contaminated Industrial Waste Water and Tolerance of Lead Metal Ion by Dominant Fungi. Research Journal of Environmental Sciences. 2015;9(4):159-168. DOI: 10.3923/rjes.2015.159.168.
- Hollos L., Novi f., Kecskemetiensis R. Annals historic-naturales Musei nationalis hungarici. 1907;5:456.
- Soni R. D., Diwan R. Evaluation of fungal diversity in certain leguminous crops of Chhattisgarh. Indian J. sci. Res. 2017;12(2):145-149.
- Mahish P. K., Tiwari K. L., Jadhav S. K. Physiochemical and Microbial studies of Paper mill Effluent, Raipur (Chhattisgarh), India. Banat’s Journal of Biotechnology. 2014;(9):57-62.
CrossRef - Deo S. S. Occuranc of fungi in Pond Water (Dumartarai Talab) at Raipur City, C.G. India. Journal of Phytology. 2011;3(4):30-34.
- Patil V. A., Mehta B. P., Deshmukh A. J. Field Evaluation of different Fungicides against Phoma Leaf Spot Disease of Indian Bean. International Journal of Pharma and Bio Sciences. 2010;6(2):1-5.
- Saju K. A., Deka T. N., Sudharshan M. R., Gupta U., Biswas A. K. Incidence of Phoma leaf spot disease of large cardamom (Amomum subulatum Roxb.) and in vitro evaluation of fungicides against the pathogen. Journal of Spices and Aromatic Crops. 2011;20(2):86–88.
- Gondal A. S., Ijaz M., Riaz K., Khan A. R. Effect of Different Doses of Fungicide (Mancozeb) against Alternaria Leaf Blight of Tomato in Tunnel. J Plant Pathol Microb. 2012;3:125. doi:10.4172/2157-7471.1000125.
CrossRef - Shirurkar D. K., Wahegaonkar N. K. Antifungal activity of selected plant derived oils and some fungicides against seed borne fungi of maize. European Journal of Experimental Biology. 2012;2(5):1693-1696.

This work is licensed under a Creative Commons Attribution 4.0 International License.





