How to Cite | Publication History | PlumX Article Matrix
S. Sivarathnakumar1,2, G. Baskar3, B. Bharathiraja4, R. Praveen Kumar2, S. Chozhavendhan2, J. Vinoth Arulraj2, K. M. Vishnuvardan2 and S. Surendar2
1Faculty of Bioengineering, Department of Biotechnology, Sathyabama University, Chennai.
2Department of Biotechnology, Arunai Engineering College, Tiruvannamalai.
3Department of Biotechnology, St. Joseph’s College of Engineering, Chennai.
4Department of Chemical Engineering, Vel Tech High Tech Dr.Rangarajan, Dr.Sakuntala Engineering College, Chennai, India.
Corresponding Author E-mail: basg2004@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2528
ABSTRACT: Prosopis juliflora, a widely available perennial plant can be an alternative source to sugar-containing feedstock, which can be considered as a prospective lignocellulosic material for bioethanol production. In the present study, bark of Prosopis juliflora was subjected to hydrothermal coupled with nitric acid pre-treatment (3%(v/v)) followed by sonication. The composition of cellulose, hemicellulose, lignin, reducing sugars and inhibitors at each stage of pre-treatment were analysed. Further, delignified lignocellulosic biomass was subjected to Simultaneous Saccharification and Fermentation (SSF) studies using Kluyveromyces marxianus (MTCC 1389) and commercial cellulase enzyme. The effect of operating parameters such as pH, temperature, substrate concentration and inoculum concentration were investigated and found to be 4.9, 41oC, 3% v/v and 5% w/v respectively. The maximum bioethanol concentration achieved by fermentation of woody stem Prosopis juliflora using the yeast was found to be 21.45g/l.
KEYWORDS: Kluyveromyces Marxianus; Lignocellulosic Material; Prosopis Juliflora SSF;
Download this article as:| Copy the following to cite this article: Sivarathnakumar S, Baskar G, Bharathiraja B, Kumar R. P, Chozhavendhan S, Arulraj J. V, Vishnuvardan K. M, Surendar S. Prosopis Juliflora Bark - An Alternate Feedstock in the Production of Bioethanol Using Thermo Tolerant Yeast Kluyveromyces Marxianus. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Sivarathnakumar S, Baskar G, Bharathiraja B, Kumar R. P, Chozhavendhan S, Arulraj J. V, Vishnuvardan K. M, Surendar S. Prosopis Juliflora Bark - An Alternate Feedstock in the Production of Bioethanol Using Thermo Tolerant Yeast Kluyveromyces Marxianus. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27694 |
Introduction
Currently, the field of biofuel production has received large incentives mainly due to the escalating prices of crude oil, depletion of fossil fuels and alarming rise of greenhouse gases.1 Among the biofuels, bio-ethanol has gained substantial interest as it provides energy security, decreases the carbon emissions and acts as closed carbon cycle. Usage of ethanol as an additive can decrease the fuel cost, it also increases octane rating and decreases harmful emissions while burning.2 Second generation biofuel production from lignocellulosic materials is considered to be a promising resource when compared to starch or sugar based biomass for it is non edible and available abundantly.3 Basically, bio-ethanol production is carried out by four stage processes which comprises of pre-treatment, Simultaneous Saccharification and Fermentation, distillation and product purification. Lignocellulosic materials such as agro biomass are being considered as prospective renewable sources for bio-ethanol production, since they contain large magnitudes of potentially fermentable sugars. The chief structural components of lignocellulosic biomass include cellulose, hemicelluloses, and lignin.4 Prosopis juliflora, a lignocellulosic biomass belonging to Leguminaceae (Fabaceae) is neither a tree nor shrub sized woody perennial plant. It is found mostly in the dry regions of India, Saudi Arabia and USA. The plant rises to a height of 3–15 metres depending on genetic variations and other environmental factors, but under favourable environmental conditions some may reach up to 20 m. The carbohydrate content in this bark of Prosopis juliflora was found to be 67.40 ± 1.6% that proves to be a suitable substrate for bioethanol production.5
The strategy behind pre-treatment has been observed as one of the most lucrative step for the conversion of lignocellulosic biomass to fermentable sugars. Auto-hydrolysis enhances the crystallinity of cellulose and hence there is an indication that delignification is been stimulated. On the other hand, acid pre-treatment involves the utilization of strong acids like hydrochloric acid, sulfuric acid and nitric acid which eliminates hemicellulose components, gives higher reaction rate and exposes cellulose for enzymatic hydrolysis.6 The production of bioethanol is carried out by two major processes namely, Separate Hydrolysis and Fermentation (SHF) and Simultaneous Saccharification and Fermentation (SSF). SSF is considered to be a better approach since it offers various benefits like diminished product inhibition, restricted operational costs and reduced contamination risks.7 Apart from economical advantages, SSF suffers a serious problem which is the difference in the temperature optima of cellulase enzyme and fermenting organism.8
Fermentable sugars are obtained from delignified biomasses by enzymatic processes where complex polysaccharides are transformed into simple monomers which involve less energy and mild environmental conditions. Enzymatic hydrolysis is carried out by cellulase enzyme which are highly specific towards the substrate. Tricoderma sp. is one among the most studied well studied cellulase producing fungal organism.9 The quantity of cellulase can be increased up to a certain extent which would enhance the yield of glucose and rate of hydrolysis simultaneously increasing the production cost. Cellulase dosage could be varied from 7 to 33 FPU/g substrate depending on type and concentration of lignocellulosic biomass used. Generally, 10-15 FPU/g substrate is used for attainment of increased level of glucose yield in a reasonable time (48-60 hours). The role of surfactant (Tween 80) during hydrolysis is its capability of altering the surface property of cellulose thereby reducing the irreversible binding of enzyme with cellulose.10
In the present study, bark of Prosopis juliflora was subjected to hydrothermal coupled with nitric acid pre-treatment (3%(v/v) followed by sonication and the composition of cellulose, hemicellulose, lignin, reducing sugars and inhibitors at each stage of pre-treatment were analysed. Further, Simultaneous Saccharification and Fermentation (SSF) studies was carried out using Kluyveromyces marxianus (MTCC 1389) and commercial cellulase along with delignified lignocellulosic biomass. The effect of operating parameters such as pH, temperature, substrate concentration and inoculum volume were investigated. The maximum bioethanol concentration achieved by fermentation of woody stem Prosopis juliflora using the yeast was found.
Materials and Methods
Collection and Analysis of Lignocellulosic Biomass
The woody stem of Prosopis juliflora was obtained from Melmalaiyanur village, Tiruvannamalai district, Tamilnadu, India. The size reduction of feed stock was done by chipping followed by milling in a knife mill. The powdered material was washed with tap water and dried overnight at 60°C. The composition of the biomass was analysed for holocellulose and lignin content.
Pre-treatment
Sequential Treatment of Woody Stem Prosopis Juliflora
The air-dried dust free woody substrate was subjected to steam in an autoclave at 121°C, 15 psi for one-hour residence time and further dried overnight at 60°C. After hydrothermal treatment, the dried sample was exposed to nitric acid (3% (v/v)) with solid to liquid ratio of 1:10 at 70°C for one hour in a heating mantle. The contents were filtered using double layered muslin cloth and the hydrolysate was washed with tap water to attain a neutral pH. The left over residue was dried to constant weight and later subjected to sonication. The auto hydrolysed and acid treated samples with distilled water was taken in a solid to liquid ratio of 1:10 (w/v) placed in glass flask. Ultrasound treatment was given at a frequency of 40 KHz, chamber temperature of 60°C for 5 min (Model: Sonics vibra cell, power 500 W, processor: 750 W, VCX series).
Combination of Treatment (Auto-Hydrolysis and Acid) Followed by Sonication of Woody Stem Prosopis Juliflora
The air dried dust free woody substrate is taken along with nitric acid (3%(v/v)) with solid to liquid ratio of 1:10 and placed in autoclave for one hour at 121°C, 15 psi. The contents were filtered using double layered muslin cloth and the hydrolysate was washed with tap water to attain a neutral pH. The left over residue was dried to constant weight and later subjected to sonication. The auto hydrolysed and acid treated samples with distilled water was taken in a solid to liquid ratio of 1:10 (w/v) placed in glass flask. Ultrasound treatment was given at a frequency of 40 KHz, chamber temperature of 60°C for 5 min (Model: Sonics vibra cell, power 500 W, processor: 750 W, VCX series).
Micro-Organism and Culture Conditions
Kluyveromyces marxianus (MTCC 1389) was procured from IMTECH, Chandigarh and was maintained in agar slopes containing (g/l): Glucose 10, yeast extract 3, Malt extract 3, Peptone 5, Agar 20, distilled water, pH was adjusted to 7.0 and temperature was maintained at 25°C. Inoculum Preparation was carried out using (w/v): Glucose 5, yeast extract 0.5, Peptone 0.5, potassium dihydrogen phosphate 0.1, distilled water and pH was adjusted to 7.0 and temperature was maintained at 25°C.
Enzyme and Surfactant
Commercial cellulase from Trichoderma reesei (ATCC 26921), Tween 80 and sodium azide where procured from HIMEDIA laboratories, Mumbai. The activity of the enzyme was measured by Filter Paper assay and expressed in terms of Filter Paper Units, FPU.11 The enzyme activity was found to be 6 FPU/mg and it was used throughout the experimentation. Enzymatic hydrolysis assays are done for pre-treated cellulosic hydrolysate of Prosopis juliflora along with cellulase was suspended in 0.05 M citrate phosphate buffer (pH 5.0)
Simultaneous Saccharification and Fermentation (SSF)
SSF utilises both enzyme and fermenting organism in a single step that enables sugar production and fermentation into bioethanol in one reactor. SSF was carried out under aerobic conditions in a 250 ml shake flask with working volume of 50 ml containing 0.05 M citrate phosphate buffer (50 mmol/l, pH 5) with pre-treated substrate which is supplemented with nutrients (g/l): yeast extract 0.5, Peptone 0.5, potassium dihydrogen phosphate 0.1. The slurry was then autoclaved at 121°C for 20 min. In addition, a dosage of commercial cellulase with 12 FPU/g of substrate and 1 ml of inoculum (OD is 0.6 at 680 nm) was loaded into the substrate mixture and operated under controlled conditions with an agitation speed of 120 rpm. A quantity of 0.005% sodium azide was introduced to avoid any microbial contamination and 1.0% (v/v) of Tween 80 was added to facilitate the enzymatic action. Samples were withdrawn from SSF media at pre-set times (0, 12, 24, 36, 48, 60, 72, 84, 96 hrs), centrifuged (10,000 rpm, 10 min) and analysed for reducing sugar and ethanol by DNS method and gas chromatography (GC) technique respectively. This method is carried out for different values of pH, temperature, substrate concentration and inoculum volume in order to estimate the optimised value for bioethanol production.
Analytical Methods
Composition of Woody Stem Prosopis Juliflora
The composition of components in woody stem Prosopis juliflora such as cellulose, hemicellulose, lignin, ash, glucose, fructose, xylose, arabinose were determined using HPLC BioRad Aminex HPX-87H column eluted with 0.01 M sulfuric acid at a flowrate of 0.6 ml/min with refractive index detector.
Reducing Sugar
The concentration of reducing sugars was estimated from pre-treated hydrolysate by Di-Nitro Salicylic Acid (DNSA) method.12
Cell Density
Biomass concentration of Kluyveromyces marxianus was measured turbidiometrically at 680 nm. Fermented broth was diluted ten times using sterile distilled water and the turbidity was detected using UV-VIS Spectrophotometer (Systronic India Private Limited).
Dry Biomass Concentration
Biomass concentration in dried conditions was carried out by taking 10 ml of culture sample, centrifuged at 10,000 g for 10 min. Supernatant was discarded and dried to a constant mass at 100°C.
Ethanol Determination
The concentration of cellulosic ethanol was estimated by GC (Perkin Elmer, Clarus 500)
with an elite-wax (cross bond-polyethylene glycol) column (30.0 m × 0.25 mm) at oven
temperature of 85°C and flame ionisation detector (FID) at 200°C. The ethanol standards
were prepared using commercial grade ethanol (Merck, Darmstadt, Germany). Nitrogen
with a flow rate of 0.5 ml/min was used as carrier gas.
Results and Discussion
The substrate which was subjected to two types of treatment processes were analysed. The combination process followed by sonication gave maximum content of cellulose (49±0.7) and hemicellulose (24±0.1) and lesser content of lignin (18±0.66). The holocellulose content was found to be better than the reported value.13
Acid pre-treatment is done by concentrated or diluted acids (usually between 0.2% and 2.5% v/v) at temperatures between 130 °C and 210 °C. Sulfuric acid is extensively used for acid pre-treatment when compared to hydrochloric acid, nitric acid and phosphoric acid.14
In the current study nitric acid (3% v/v) was used which gave 75% of reducing sugar concentration at 70°C with mild charring and when the temperature was increased to 90°C complete charring occurred, unlike sulphuric acid getting charred at 70°C.14 The combination process followed by sonication of woody stem Prosopis juliflora was found to contain 35.5g/l cellulosic hydrolysate which is subjected for Simultaneous Saccharification and Fermentation using pure cellulase enzyme and thermo tolerant yeast Kluyveromyces marxianus.
Table 1: Composition of woody stem Prosopis juliflora before and after pre-treatment
| Sample | Weight (gm) | Cellulose | Hemicelluloe | Lignin | Glucose | Fructose | Xylose | Arabinose | Ash |
| 1 | 50 | 40±0.3 | 22±0.0 | 22±0.01 | 2±0.22 | 3±0.01 | 12±0.61 | 1±0.05 | 0.24±0.00 |
| 2 | 45 | 48±0.5 | 24±0.3 | 21±0.75 | 3±0.33 | 3±0.00 | 11±0.66 | 1±0.98 | 0.21±0.23 |
| 3 | 35.5 | 43±0.2 | 21±0.8 | 20±0.15 | 3±0.14 | 2±0.71 | 11±0.62 | 0.9±0.15 | 0.21±0.11 |
| 4 | 33 | 42±0.2 | 21±0.8 | 20±0.15 | 3±0.14 | 2±0.71 | 11±0.62 | 0.9±0.15 | 0.21±0.11 |
| 5 | 41 | 48±0.7 | 23±0.1 | 19±0.66 | 2±0.75 | 3±0.09 | 13±0.31 | 1±0.65 | 0.22±0.54 |
| 6 | 40 | 49±0.7 | 24±0.1 | 18±0.66 | 3±0.75 | 3±0.09 | 13±0.31 | 1±0.65 | 0.21±0.54 |
1: Raw sample, 2: Auto hydrolysed, 3: Auto hydrolysed sample kept in heating mantle at 70°C using nitric acid 3%(v/v), 4: Auto hydrolysed sample kept in heating mantle at 70°C using nitric acid 3%(v/v) subjected to sonication, 5: Combination of auto hydrolysis and nitric acid 3%(v/v) in autoclave, 6: Combination of auto hydrolysis and nitric acid 3%(v/v) sample subjected to sonication.
The cellulosic hydrolysate of bark of Prosopis juliflora was subjected to SSF process along with cellulase enzyme and thermo tolerant yeast Kluyveromyces marxianus by varying the concentration of the substrate (3%, 5% and 7% w/v). The consumption of sugars was observed initially during 12 hours but the growth and metabolism of the organism was found to increase steadily after 20 hours. In case of 5.0% (w/v) substrate concentration, at 48 hours of incubation period 60% (12.25 g/l) of reducing sugar has been converted into 12.75 g/l of bioethanol with a biomass concentration of 6.25 g/l. The maximum bioethanol concentration achieved was 21.45g/l at around 96hrs of incubation. As substrate concentration is increased further to 7.0% (w/v), it decreases the yield from hydrolysis due to bioethanol inhibition and extent of substrate inhibition depends on ratio of substrate to cellulase loaded. Increased bioethanol production at lower substrate concentration would be due to lower glucose accumulation by the end of fermentation process.15
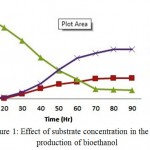 |
Figure 1: Effect of substrate concentration in the production of bioethanol
|
For the optimum concentration of substrate at 5%(w/v), the pH (4.6, 4.9, 5.2 and 5.5) was varied. The bioethanol concentration increased steadily after 24 hrs of incubation wherein at around 60 hours of culture the bioethanol concentration and biomass concentration has reached up to 75% (17.25 g/l, 7.45g/l) of the total concentration for an optimum pH of 4.9 whose substrate concentration was about 25-30% (9.25 g/l) of the initial condition. The maximum bioethanol concentration achieved was 21.25g/l at around 96hrs of incubation. When pH of the medium increases to 5.5 or decreases to 4.6, it affects the enzymatic hydrolysis during Simultaneous Saccharification and Fermentation process leading to decrease in the production of bioethanol. The use of ricotta whey in the production of bioethanol by SSF process in the presence of Kluyveromyces marxianus CCT 7735 (UFV-3) has yielded 49.65 g/l of bioethanol at a pH of 5.0. When pH is increased to 5.5 or 4.5 the bioethanol production is significantly low.1
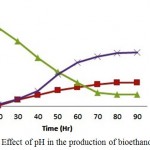 |
Figure 2: Effect of pH in the production of bioethanol
|
The pre-treated woody biomass Prosopis juliflora when subjected to Simultaneous Saccharification and Fermentation using cellulase enzyme and thermo tolerant yeast Kluyveromyces marxianus was performed with varying temperature (33°C, 37°C, 41°C and 45°C). The concentration of bioethanol and biomass intensified after 24 hours of incubation time wherein at 48 hours it reported 60% (12.55 g/l and 6.5 g/l) of the total concentration for an optimum temperature 41°C. The maximum bioethanol concentration achieved was 21.30g/l at around 96hrs of incubation. The optimal temperature for enzymatic hydrolysis and fermentation are specific, temperature becomes a limiting factor for SSF. The maximum bioethanol obtained from taro waste using K. marxianus K21 at 40°C was found to be 43.46 g/l but, as temperature escalated to 50°C the bioethanol production dropped down to 19.15g/l. It clearly states that though Saccharification takes place at around 50°C effectively, bioethanol production declines at this temperature due to accumulation of glucose during SSF process eventually leading to death of yeast under high temperature.16
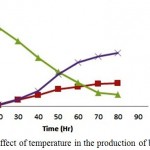 |
Figure 3: Effect of temperature in the production of bioethanol
|
The delignified biomass of Prosopis juliflora was exposed to Kluyveromyces marxianus in the presence of cellulase enzyme by SSF process and inoculum concentrations were varied (1%,3% and 5% (v/v)). During initial stages of fermentation, organism utilises the sugars and generates good amount of biomass. After 48 hours of incubation time the bioethanol and biomass concentration intensifies to 60 % (11.75g/l and 5.35 g/l ) of total concentration for an optimum inoculum concentration of 3%(v/v). Maximum bioethanol concentration was found to be 21.30 g/l at around 96 hrs of incubation. The production of bioethanol from carob fruit extracts using Saccharomyces cerevisiae was found to be around 42g/l for an inoculum concentration of 3%(v/v).17 Bioethanol production from waste potato mash using Saccharomyces cerevisiae was found to be around 27g/l for an inoculum concentration of 3%(v/v).18
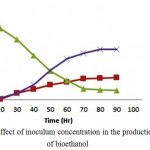 |
Figure 4: Effect of inoculum concentration in the production of bioethanol
|
Conclusion
The bark of Prosopis juliflora would be a promising alternative lignocellulosic material in the production of bioethanol. The combination of autohydrolysis coupled with nitric acid pretreatment has given a wide variety of information wherein the exposure of cellulose for enzymatic hydrolysis is commendable. Though the yield of bioethanol by using Kluyveromyces marxianus found to be low, it can survive at high temperatures as well as in low pH. However Saccharomyces cerevisiae yields better bioethanol for 3% inoculum concentration but cannot with stand high temperatures (>45°C) or (pH ≤5.0) In case of SSF operation, Kluyveromyces marxianus would a gifted organism in the production of bioethanol.
Funding Sources
No funding has been procured from any government agencies.
Conflict of Interest
Authors declares there are no conflict of interest.
Reference
- Gonçalves P. F., da Silveira F. A ., Cristina R. V. d., Leonardo H. A. G., Hermano R. S. D., Ivo J. R. J., Gomes L. F., Maria F. L. P and Batista W. d. Optimizing Ethanol Production by Thermotolerant Kluyveromyces marxianus CCT 7735 in a Mixture of Sugarcane Bagasse and Ricotta Whey. Food Sci. Biotechnol. 2015;24(4):1421-27.
CrossRef - Praveen R. k., Bharathiraja B., Pragadeesh K., Chozhavendhan S and William A. J. Utilization of cassava sago waste for bioethanol production by co-fermentation of starch degrading bacteria and Saccharomyces cerevisiae. Asian Jr. of Microbiol. Biotech. Env. Sc. 2014;16(3):627-632.
- García-Aparicio M. P., Oliva J. M., Manzanares P., Ballesteros M., Ballesteros I., González A., Negro M. J. Second-generation ethanol production from steam exploded barley straw by Kluyveromyces marxianus CECT 10875.Fuel. 2011;90:1624–30.
CrossRef - GOSHIMA T., TSUJI M., INOUE H., YANO S., HOSHINO T and MATSUSHIKA A. Bioethanol Production from Lignocellulosic Biomass by a Novel Kluyveromyces marxianus Strain. Biosci. Biotechnol. Biochem. 2013;77(7):1505–10.
CrossRef - Sivarathnakumar S., Baskar G., Praveenkumar R.,Bharathiraja B. Simultaneous Saccharification and Fermentation of woody stem Prosopis juliflora by Zymomonas mobilis for the production of cellulosic ethanol, Int. J. Materials and Product Technology. 2017;55(1/2/3):236-53.
- Balat M. Production of bioethanol from lignocellulosic materials via the biochemical pathway. A review, Energy Conversion and Management. 2011;52:858–75.
CrossRef - Romaní A., Garrote G., Carlos J. P. Bioethanol production from autohydrolyzed Eucalyptus globulus by Simultaneous Saccharification and Fermentation operating at high solids loading. Fuel. 2012;94:305-12.
CrossRef - Kádár Z. s., Szengyel Z. s., Réczey K. Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Industrial Crops and Products. 2004;20:103–10.
CrossRef - Sarkar N.,Kumar S. G., Bannerjee S., Aikat K. Bioethanol production from agricultural wastes: An overview. Renewable Energy. 2012;37:19-27.
CrossRef - Sun Y., Cheng J. Hydrolysis of lignocellulosic materials for ethanol production a review. Bioresour. Technol. 2002;83:1–11.
CrossRef - Ghose T. S. Measurement of cellulase activities. Pure Applied Chemistry. 1987;59:257–68.
CrossRef - Miller G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar.Analytical Chemistry. 1959;31(3):426–28.
- Shaik N., Srilekha Y. K., Sateesh L., Manikyam A., Desai L. S., Rao V. Selection of the best chemical pre-treatment for lignocellulosic substrate Prosopis juliflora. Bioresour. Technol. 2013;13:542–49.
- Sarkar N., Ghosh S. K., Bannerjee S., Aikat K. Bioethanol production from agricultural wastes: An overview. Renewable Energy. 2012;37:19-27.
CrossRef - Narra M., Jisha P. J., Balasubramanian V. Simultaneous Saccharification and fermentation of delignified lignocellulosic biomass at high solid loadings by a newly isolated thermotolerant Kluyveromyces sp. for ethanol production. Bioresour. Technol. 2015;179:331-8.
CrossRef - Wei-Hao W. u., Wei-Chun H., Kai-Yin L. o., Yen-Hui C., Hou-Peng W.,Kuan-Chen C. Bioethanol Production from Taro Waste Using Thermo-tolerant Yeast Kluyveromyces marxianus K21. Bioresour. Technol. 2016,201:27-32.
CrossRef - Turhan I., Demirci A., Karhan M. Ethanol production from carob extract by Saccharomyces cerevisiae. Bioresource Technol. 2010;101:5290–5296.
CrossRef - Izmirlioglu G & Demirci A. Ethanol production from waste potato mash by using Saccharomyces cerevisiae. Appl. Sciences. 2012;2(4):738-753.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





